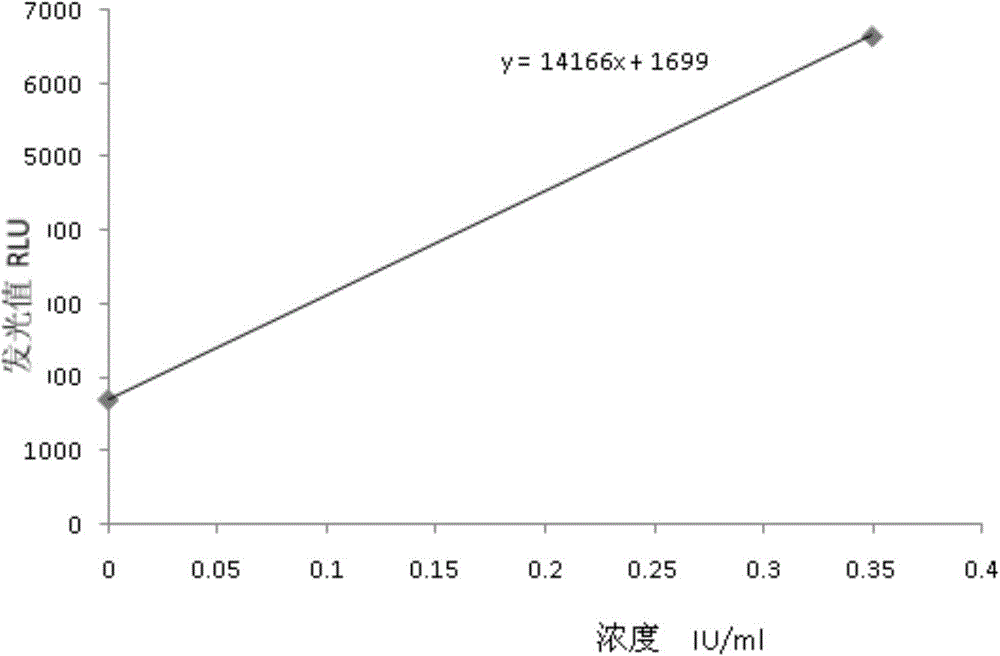
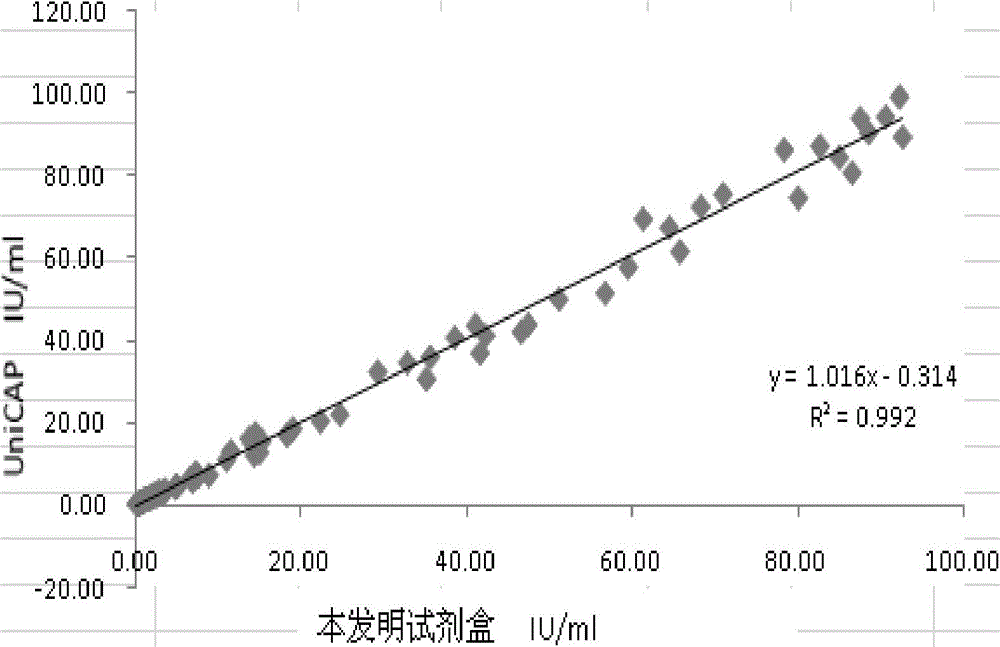
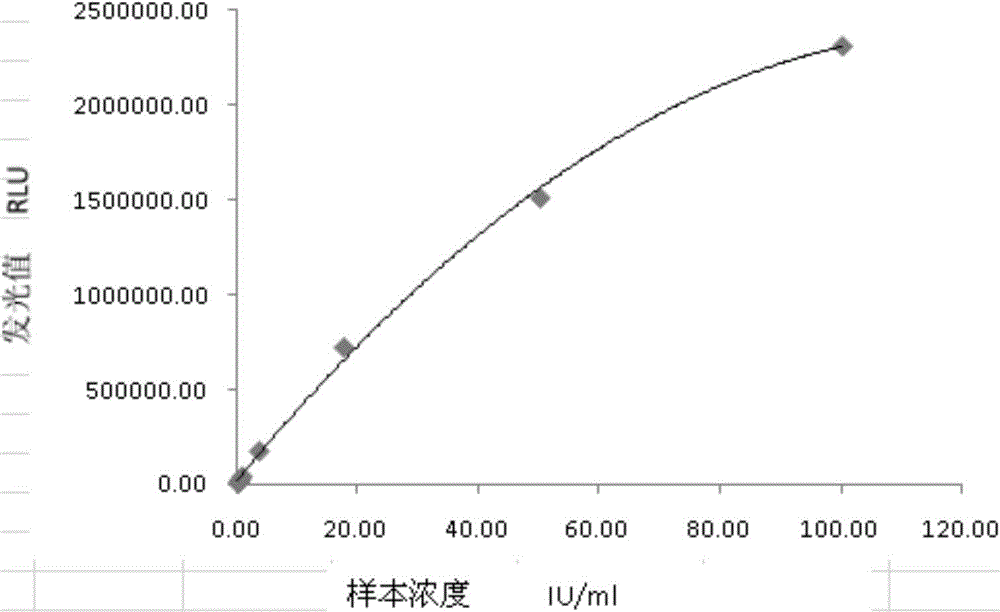
A chemiluminescent quantitative detection kit for inhaled allergens and its preparation method and detection method
A quantitative detection and chemiluminescence technology, which is applied in the field of immunoassay, can solve the problems of suffering, high risk and poor substitution of the subjects, and achieve the effect of simple operation, time saving, wide detection range and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of kit
[0034] (1) Preparation of magnetic separation reagents:
[0035] Materials and Instruments:
[0036] 1. Magnetic particle: Contains carboxyl group (COOH) active group, the carboxyl group content per gram (g) magnetic particle (dry weight) is 1 millimole (mmol), has superparamagnetism, and the diameter is 1 μm.
[0037] 2. Inhalation of allergens: Dissolve 10 mg of freeze-dried dust mite allergens in 2 ml of phosphate buffer (pH7.2, 0.02M) to obtain a solution of allergens with a concentration of 5 mg / ml. Put the resulting solution into a dialysis bag and fasten it After the dialysis bag, dialyze with 500ml of phosphate buffer at 4°C for 4-8 hours, change the solution 3-4 times, collect the dialyzed product, and the purity of the allergen protein is 90%.
[0038] 3. 2-Morpholineethanesulfonic acid (MES), carbodiimide (EDC), trishydroxymethylaminomethane (TRIS) and other reagents should be chemically pure.
[0039] Steps:
[0040...
Embodiment 2
[0055] Embodiment 2: the implementation of detection and the evaluation of detection effect:
[0056] (1) Implementation of testing
[0057] Materials and Instruments:
[0058] 1. Magnetic separation reagent and enzyme-labeled reagent, prepared by Example 1.
[0059] 2. IgE calibrators, quality control products, luminescent substrates, cleaning solutions, and specific IgE assay kits (fluorescence method) are produced by Pharmacia.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com