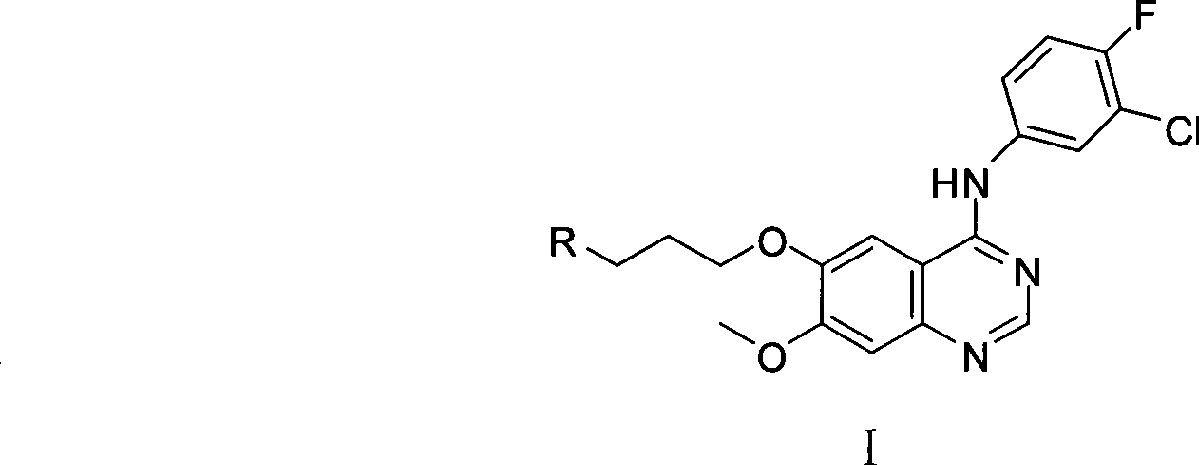
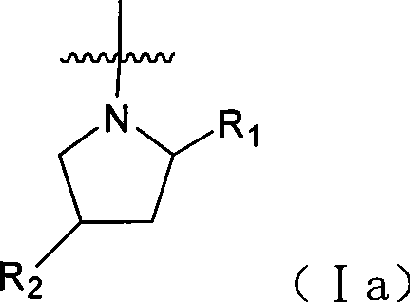
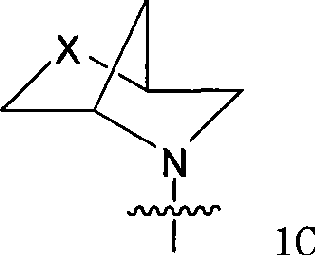
Amino-quinazoline derivative with antineoplastic activity and its salts
A technology of aminoquinazoline and methoxyquinazoline, applied in the field of chemistry, can solve the problems of low efficiency of non-small cell lung cancer, unsatisfactory clinical effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 4-(3'-chloro-4'-fluoroanilino)-6-(3'-N-(4'-hydroxypiperidinyl))propoxy)-7-methoxyquinazole Preparation of morphine (compound 7)
[0027]
[0028] Compound 7
[0029] Preparation of the first step 6,7-dimethoxy-4(3H)-quinolinone (compound 1)
[0030]
[0031] Compound 1
[0032] The starting material 4,5-dimethoxy-2-aminobenzoic acid (79.0 g, 400 mmol) and 95 ml of formamide were heated to reflux at 180° C. and stirred for 5 h. Stop heating, add 200ml of cold water to the hot solution, stir at room temperature for 10min, let stand for 50min, filter, wash the filter cake with 3×100ml of water, and dry in vacuo to obtain 11.9g of off-white solid powder with a yield of 14.2%. 1 HNMR (DMSO-d 6 , 400Hz), δ 12.14(s, 1H), 7.89(s, 1H), 7.47(s, 1H), 7.13(s, 1H), 3.83(s, 6H); MS(EI) m / z 207(M +1) + .
[0033] Preparation of the second step 7-hydroxy-7-methoxy-4(3H)-quinolinone (compound 2)
[0034]
[0035] Compound 2
[0036] Compound 1 (26.5g, 128.6mm...
Embodiment 2
[0058] 4-(3'-chloro-4'-fluoroanilino)-6-(3'-N-(3'-hydroxypiperidinyl))propoxy)-7-methoxyquinazoline (compound 8) Preparation
[0059]
[0060] Compound 8
[0061] Compound 6 (100mg, 0.313mmol), N-(3'-chloropropyl)-3-hydroxypiperidine (78mg, 0.438mmol), in a flask, add 250mg K 2 CO 3 , 47mgNaI, 5ml DMF, placed in an oil bath at 110°C for 4 hours, stopped heating, cooled naturally to room temperature, then added 30ml water and 30ml ethyl acetate to the reaction solution, separated the liquid, extracted the aqueous phase with 30ml ethyl acetate, The organic phases were combined, washed twice with 50 ml of water, then washed with saturated saline solution, dried over anhydrous magnesium sulfate, and rotary evaporated to obtain 78.0 mg of brown solid powder with a yield of 70.5%. 1 HNMR (CDCl 3 )δ 8.614(s,1H), 8.543(s,1H), 8.008-7.960(m,1H), 7.676-7.668(m,1H), 7.438(s,1H), 7.213(s,1H), 7.115( t, J=8.8Hz, 1H), 4.338-4.317(m, 1H), 4.215-4.197(m, 1H), 3.971(s, 4H), 3.481(s, 2H...
Embodiment 3
[0063] 4-(3'-chloro-4'-fluoroanilino)-6-(3'-N-(3'-hydroxypyrrolidinyl))propoxy)-7-methoxyquinazoline (compound 9 )
[0064]
[0065] Compound 9
[0066] Compound 6 (100mg, 0.313mmol), N-(3'-chloropropyl)-3-hydroxypyrrolidine (72mg, 0.438mmol), in a flask, add 250mg K 2 CO 3 , 47mgNaI, 5ml DMF, placed in an oil bath at 110°C for 4 hours, stopped heating, cooled naturally to room temperature, then added 30ml water and 30ml ethyl acetate to the reaction solution, separated the liquid, extracted the aqueous phase with 30ml ethyl acetate, The organic phases were combined, washed twice with 50 ml of water, then washed with saturated saline solution, dried over anhydrous magnesium sulfate, and rotary evaporated to obtain a brown solid powder, which was passed through a silica gel column, and the eluent was PE:EA=2:1 to obtain 76 mg solid, yield 66.5%. 1 HNMR (CDCl 3 )δ 8.617(s, 1H), 8.415(s, 1H), 7.966-7.944(dd, J=6.4Hz, 2.4Hz, 1H), 7.639-7.601(m, 1H), 7.478(s, 1H), 7.203 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com