Humanized EGFR antibodies
A humanized antibody and antibody technology, applied in the direction of antibodies, antineoplastic drugs, chemical instruments and methods, etc., can solve the problems of low HAMA response and low immunogenicity of humanized antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] Example 1: Humanization of the Murine Heavy and Light Chain Variable Regions of Anti-EGFR Antibodies
[0123] The coding nucleic acid sequences of the heavy chain and light chain variable regions (VH, SEQ ID NO:4 and VL, SEQ ID NO:8) of the monoclonal antibody targeting the epitope in the extracellular ligand binding domain of human EGFR1 The genomic sequences of the human constant γ1 region (CH) and the human constant κ region (CL) are each linked.
[0124] Based on these chimeric clones, humanized antibodies were constructed. To this end, point mutations were introduced into the nucleic acid sequences of the murine framework regions of VH and VL to generate the corresponding human framework regions. The human framework regions of interest are selected from human germline antibody libraries. In particular, the most related framework regions were selected from the library based on their overall sequence similarity and CDR loop classification. Considering all the data...
Embodiment 2
[0129] Example 2: Binding of humanized antibody variants to immobilized EGFR
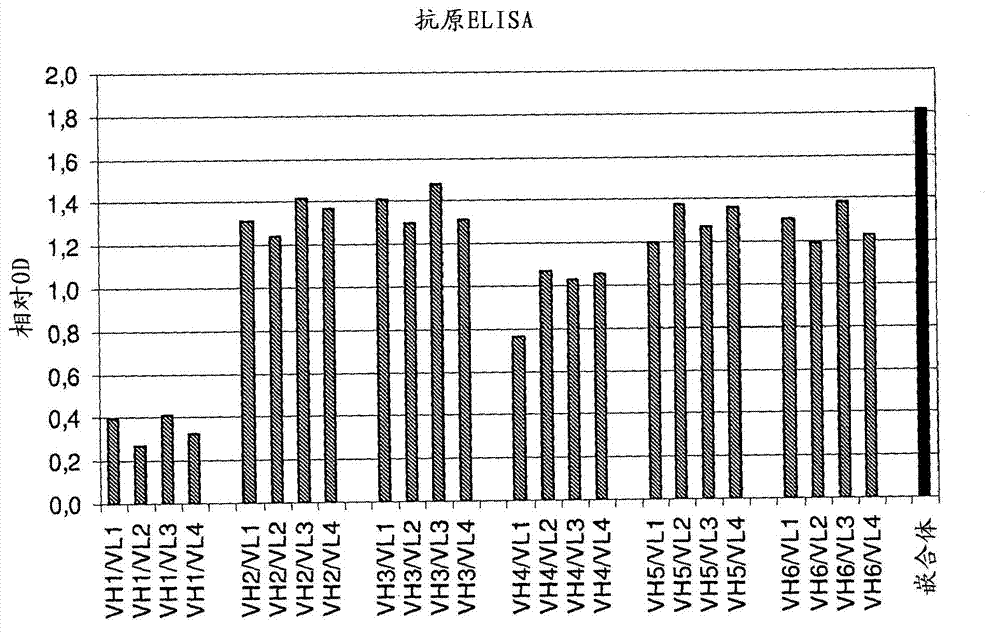
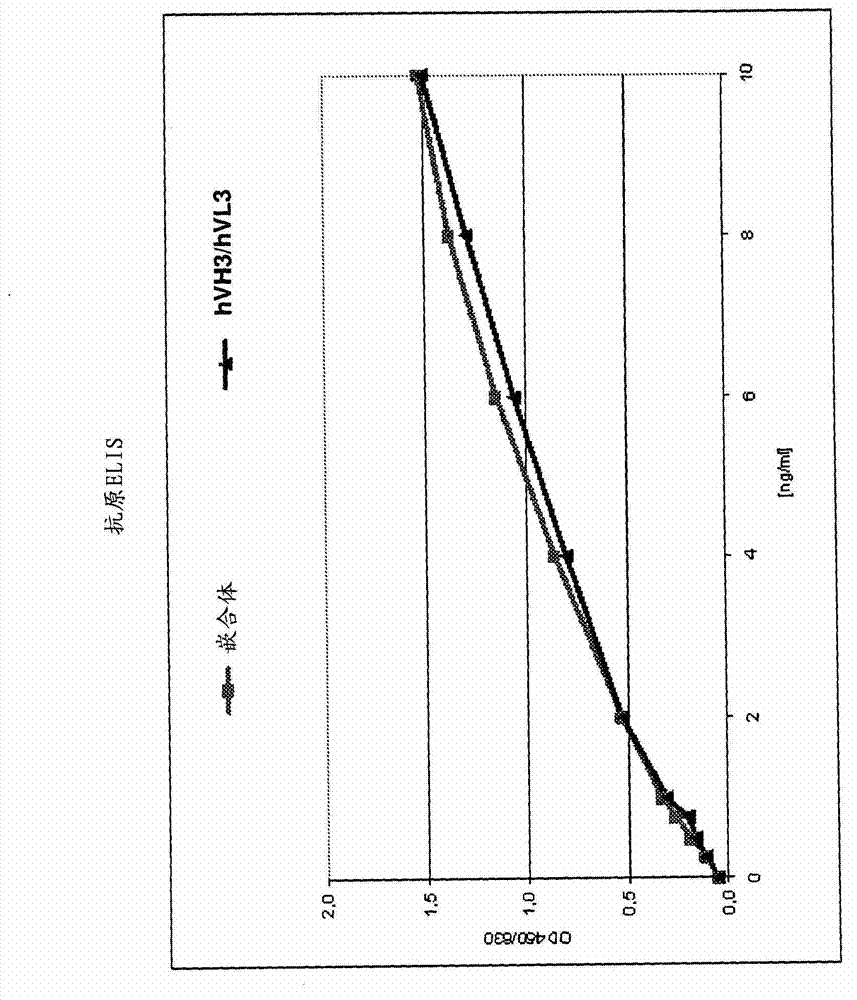
[0130] After expression of the different constructs in COS cells, the titers of the humanized antibody variants were determined and their concentrations adjusted. Then, humanized antibodies were screened in antigen ELISA. Exemplary results of one round of screening are shown in figure 1 middle. All variants showed significant binding to the antigen. Good antigen binding was observed for humanized antibody variants comprising a heavy chain variable region selected from VH2, VH3, VH5 and VH6. In particular, the variant VH3 / VL3 showed good results. In addition, antibodies comprising heavy chain variable regions VH7 or VH8 also showed good antigen binding. figure 2 A direct comparison of the chimeric antibody and the humanized antibody variant hVH3 / hVL3 in antigen ELISA is shown in .
Embodiment 3
[0131] Example 3: Binding of humanized antibody variants to different EGFR expressing cells
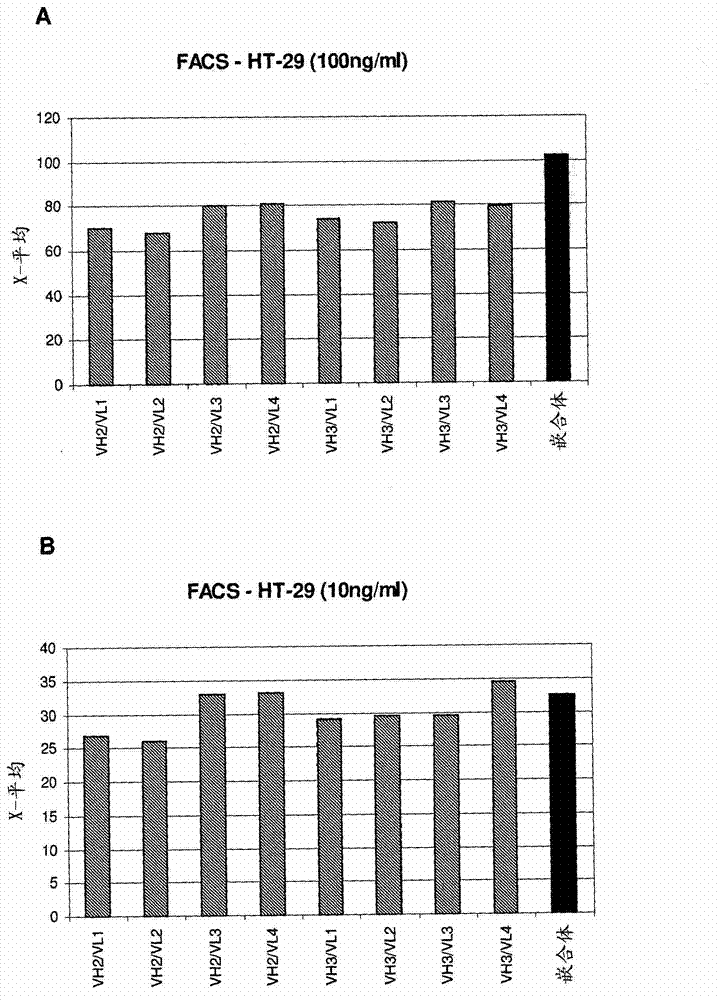
[0132] Using IgG antibodies containing these humanized heavy and light chain variable regions at different concentrations, FACS experiments using HT-29 cells, LS174.T cells or DU145 cells were performed. Figures 3 to 7 Binding of selected antibody variants is shown in . It was demonstrated that the humanized antibodies, in particular the humanized antibody variant VH3 / VL3, have comparable antigen binding properties to those of the chimeric antibodies from which they were generated. In addition, VH7 and VH8 antibody variants also showed good binding to these cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com