Fusion protein based on anti-EGFR single-chain antibody and arginine nonamer, and applications of fusion protein
An arginine nonamer and fusion protein technology, which is applied to fusion proteins and application fields based on anti-EGFR single-chain antibody and arginine nonamer, can solve problems such as non-ideal curative effect, and achieve good application prospects and preparation. Simple and convenient, reduced size effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be further described in detail below in conjunction with specific embodiments, which are explanations of the present invention rather than limitations.
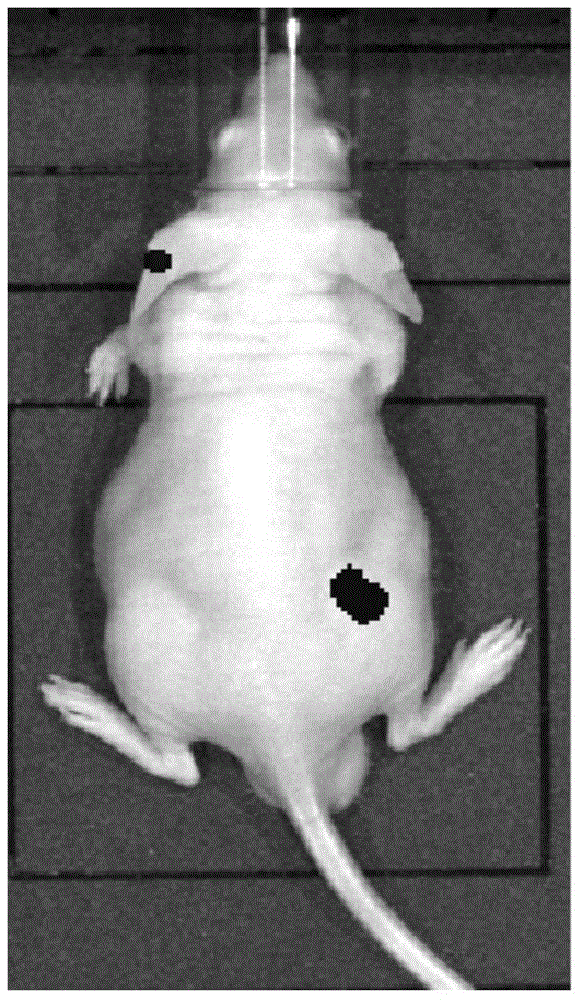
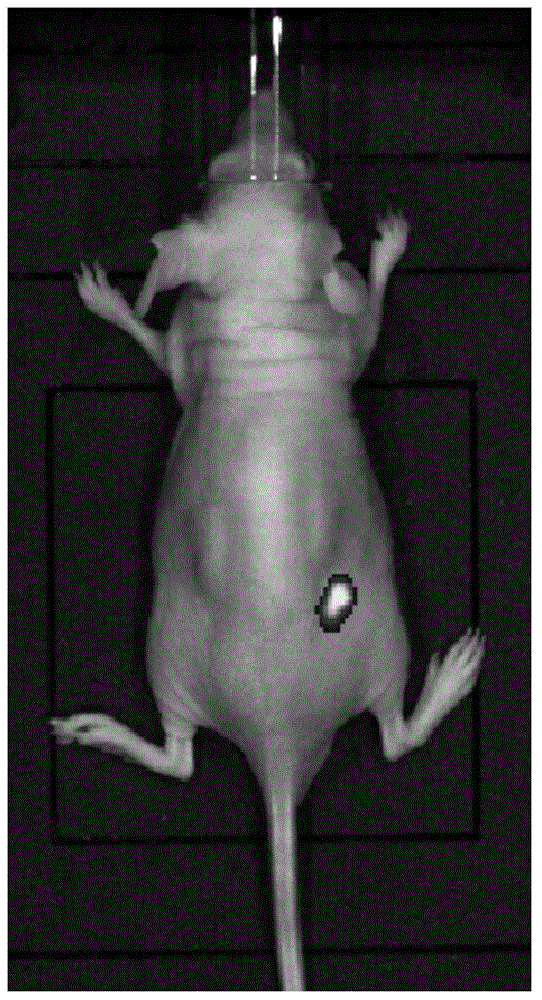
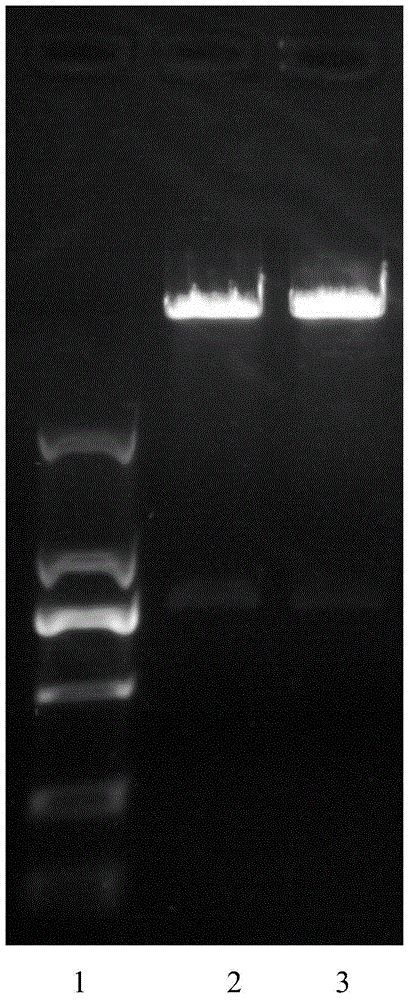
[0032] 1. Construction and preparation of anti-EGFR single-chain antibody and fusion protein
[0033] (1) Sequence construction
[0034] In order to improve the function of the anti-EGFR antibody, the present invention uses the recombinant antibody single chain to reduce the size of the antibody protein, and facilitates the expression and internalization of the recombinant protein on the basis of exerting its specific binding. The constructed EGFR single-chain antibody is composed of a linking peptide connecting the heavy chain variable region and the light chain variable region. The activity after fusion expression is taken as an important basis.
[0035] The amino acid sequence of the anti-EGFR single-chain antibody constructed in the present invention is as follows (SEQ.ID.NO.1):
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com