Paclitaxel immune nano liposome and preparation method and application thereof
A technology of nano-liposome and nano-lipid, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of PEGylated paclitaxel nanoliposomes
[0025] Cholesterol chloroformate was dissolved in anhydrous chloroform, cooled in ice, and excess N,N-dimethylethylenediamine solution was added dropwise to prepare DC-Chol. Add DC-Chol, POPC, DSPE-PEG and DSPE-PEG-MAL into an eggplant-shaped bottle in equal volumes at a molar ratio of 1:1:0.05:0.01, and blow dry by rotary evaporation at 30°C. Paclitaxel dissolved in an organic solvent (distilled water: chloroform = 1:2) was added, vortexed, and placed in an ultrasonic water bath at 20° C. for 5 minutes, so that the liposomes encapsulated the paclitaxel to form PEGylated paclitaxel nanoliposomes. In the same way, ultrapure water was used instead of paclitaxel to prepare blank nanoliposomes as a negative control in the experiment. The obtained PEGylated paclitaxel nanoliposomes and blank nanoliposomes were sequentially passed through 400, 200, 100 and 80 nm microporous membranes under the action of a film...
Embodiment 2
[0026] Example 2: Preparation of PEGylated paclitaxel immune nanoliposomes
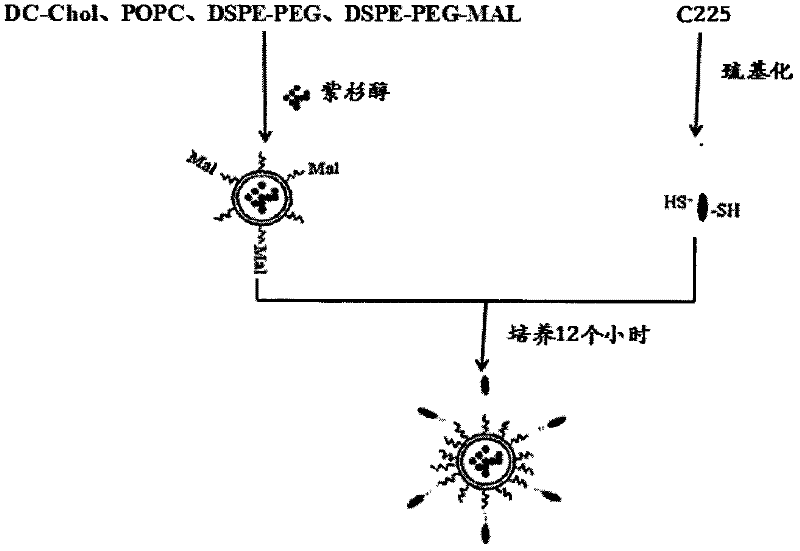
[0027] (1) Preparation of thiolated antibody
[0028] The EGFR antibody C225 was thiolated according to the experimental procedure of Traut's kit, and a standard curve was drawn.
[0029] (2) Preparation of PEGylated paclitaxel immune nanoliposomes
[0030] The paclitaxel-coated nanoliposomes were resuspended with 2 ml of ultrapure water, 1 ml of thiolated antibody C225 was added, and the coupling reaction was carried out under nitrogen protection at room temperature for 12 hours. The reaction product was passed through a SwpharoseCL-4B column to separate the unlinked antibody and the PEGylated paclitaxel nanoliposomes linked to the antibody, and the eluate was measured for fluorescence, and the elution curve was drawn. The schematic diagram of the preparation process of PEGylated paclitaxel immune nanoliposomes is as follows figure 1 shown.
[0031] The particle size distribution of the PEGylated...
Embodiment 3
[0032] Example 3: PEGylated Paclitaxel Immune Nanoliposomes for Nude Mice Experiments in the Treatment of Primary Liver Cancer
[0033] Experimental nude mice weighing 20g±1g, all female, were inoculated with 2.5×10 SMMC-7721 cells 6 One per mouse, when the tumor grows to 5-10mm, it will be divided into random groups, 5 in each group, respectively injected with PEGylated paclitaxel nanoliposomes, PEGylated paclitaxel nanoliposomes, and paclitaxel, and the total content of paclitaxel is the same. treat. Injected intraperitoneally once a day for 10 consecutive days.
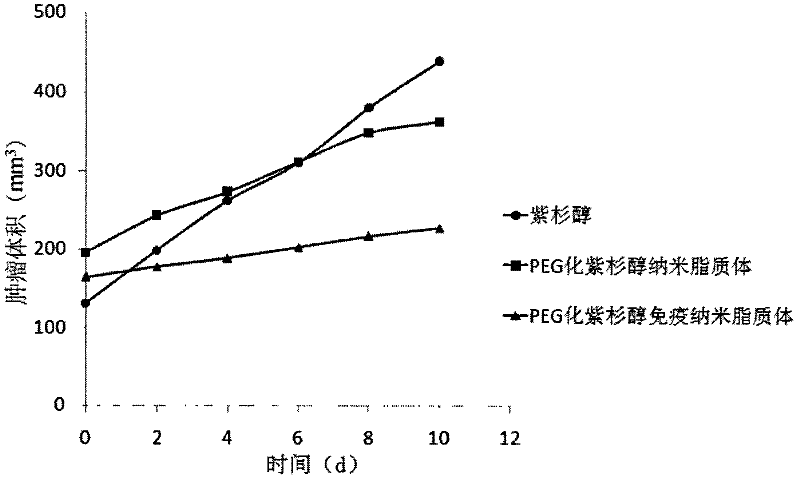
[0034] Observe curative effect:
[0035] (1) Efficacy curve: the tumor volume was measured every 2 days, expressed as tumor volume (mm 3 ) is the ordinate, the number of days of treatment is the abscissa, and the curative effect curve is drawn, such as figure 2 shown.
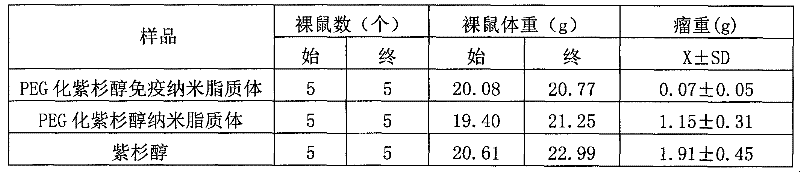
[0036] (2) Comparison of tumor suppression ability:
[0037] All the mice were sacrificed 24 hours after drug withdrawal, the tumor mass was d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com