Targeted adriamycin-loaded magnetic nanoparticles and preparation method and application
A magnetic nanoparticle, targeted modification technology, applied in the field of anti-tumor photothermal therapy, can solve the problem of lack of specific targeting, achieve good target recognition ability, excellent photothermal killing function, good magnetic resonance imaging effect of function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of target-modified magnetic nanoparticles loaded with doxorubicin (Fe 3 o 4 @PDA-PEG-EGFR-DOX) preparation method, comprises the following steps:
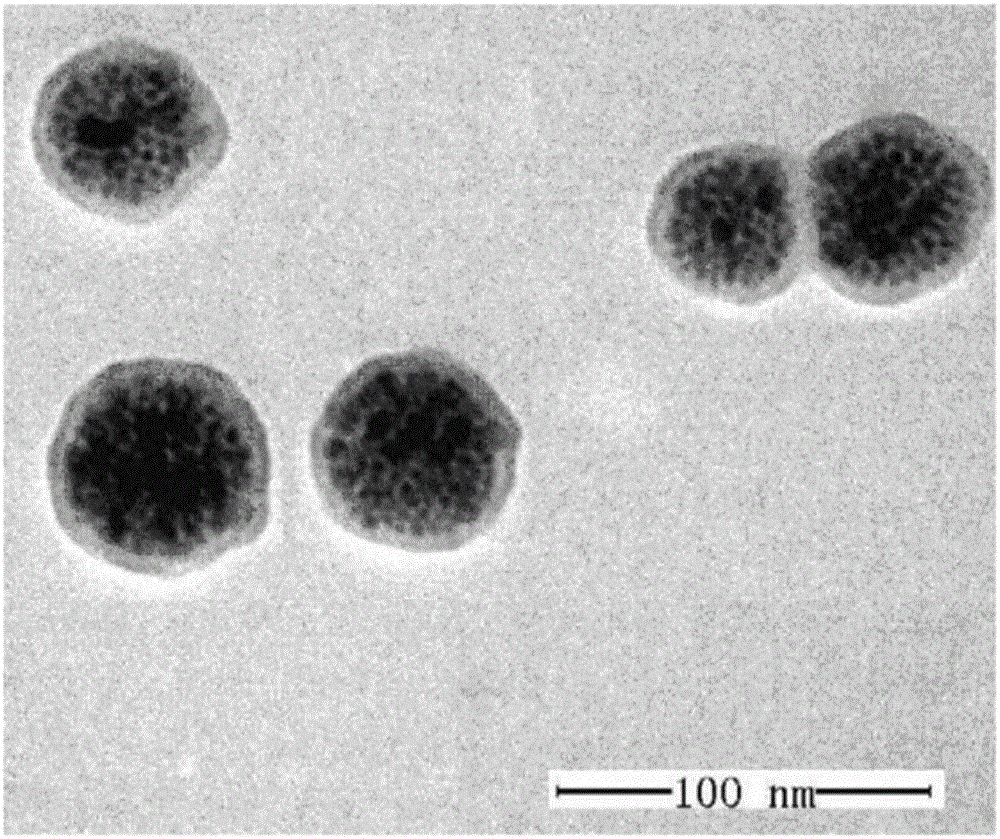
[0038] (1) 2mmol of FeCl 3 Dissolve in a mixed solution of 4mL ethylene glycol and 16mL monocarboxydiethylene glycol, stir for 0.5h, add 2g of polyvinylpyrrolidone to the above solution, heat the solution to 120°C to obtain a transparent yellow solution, after 1h, stop Heat and add 1.5g of sodium acetate, continue to stir for 0.5h, transfer the solution to a 25mL reactor, seal the reactor and heat it to 200°C, continue the reaction for 12h, stop heating and cool the reactor to room temperature to obtain Fe 3 o 4 The nanoclusters were washed three times with ethanol and water, and dissolved in Tris buffer;
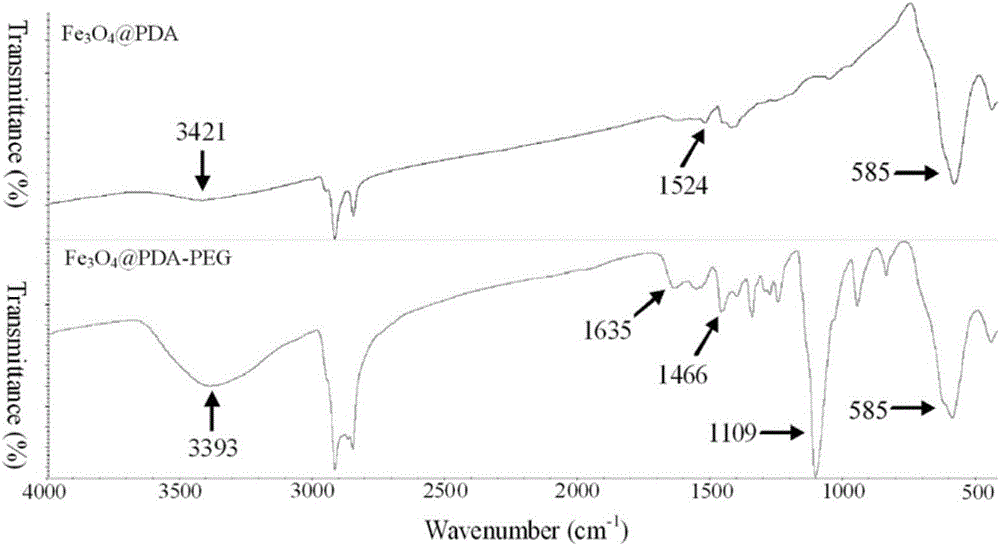
[0039] (2) the Fe-containing material prepared in step (1) 3 o 4 The Tris buffer of the nanoclusters was adjusted to pH 8.5, and 0.03M dopamine solution was slowly added, reacted at room temperature for 3 ho...
Embodiment 2
[0044] Include the following steps:
[0045] (1) 2mmol of FeCl 3 Dissolve in a mixed solution of 4mL ethylene glycol and 16mL monocarboxydiethylene glycol, stir for 0.75h, add 2g of polyvinylpyrrolidone to the above solution, and heat the solution to 120°C to obtain a transparent yellow solution. After 1.5h, Stop heating and add 1.5g sodium acetate, continue to stir for 0.75h, transfer the solution to a 25mL reactor, seal the reactor and heat it to 200°C, continue the reaction for 14h, stop heating and cool the reactor to room temperature to obtain Fe 3 o 4 The nanoclusters were washed three times with ethanol and water, and dissolved in Tris buffer;
[0046] (2) the Fe-containing material prepared in step (1) 3 o 4 Adjust the pH of the nanocluster Tris buffer to 8.5, slowly add 0.03M dopamine solution, react at room temperature for 4.5h until the color turns black, centrifuge and wash with deionized water for 3 times, and vacuum-dry the precipitate at 55°C to obtain dopa...
Embodiment 3
[0051] Include the following steps:
[0052] (1) 2mmol of FeCl 3 Dissolve in a mixed solution of 4mL ethylene glycol and 16mL monocarboxydiethylene glycol, stir for 1h, add 2g of polyvinylpyrrolidone to the above solution, heat the solution to 120°C to obtain a transparent yellow solution, stop heating after 2h And add 1.5g of sodium acetate, continue to stir for 1h, transfer the solution to a 25mL reactor, seal the reactor and heat it to 200°C, continue the reaction for 16h, stop heating and cool the reactor to room temperature, the obtained Fe 3 o 4 The nanoclusters were washed three times with ethanol and water, and dissolved in Tris buffer;
[0053] (2) the Fe-containing material prepared in step (1) 3 o 4 Adjust the pH of the nanocluster Tris buffer to 8.5, slowly add 0.03M dopamine solution, react at room temperature for 6 hours until the color turns black, centrifuge, wash with deionized water 3 times, and vacuum-dry the precipitate at 60°C to obtain dopamine-coated...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com