Combination Therapy Using Anti-EGFR and Anti-HER2 Antibodies
a technology of anti-egfr and anti-her2 antibodies, which is applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of little information available regarding antibody efficacy, the difficulty of treating adenocarcinomas of the pancreas, and the prognosis remains poor, so as to prevent the dimerization of egfr, the effect of confirming the importance of communication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
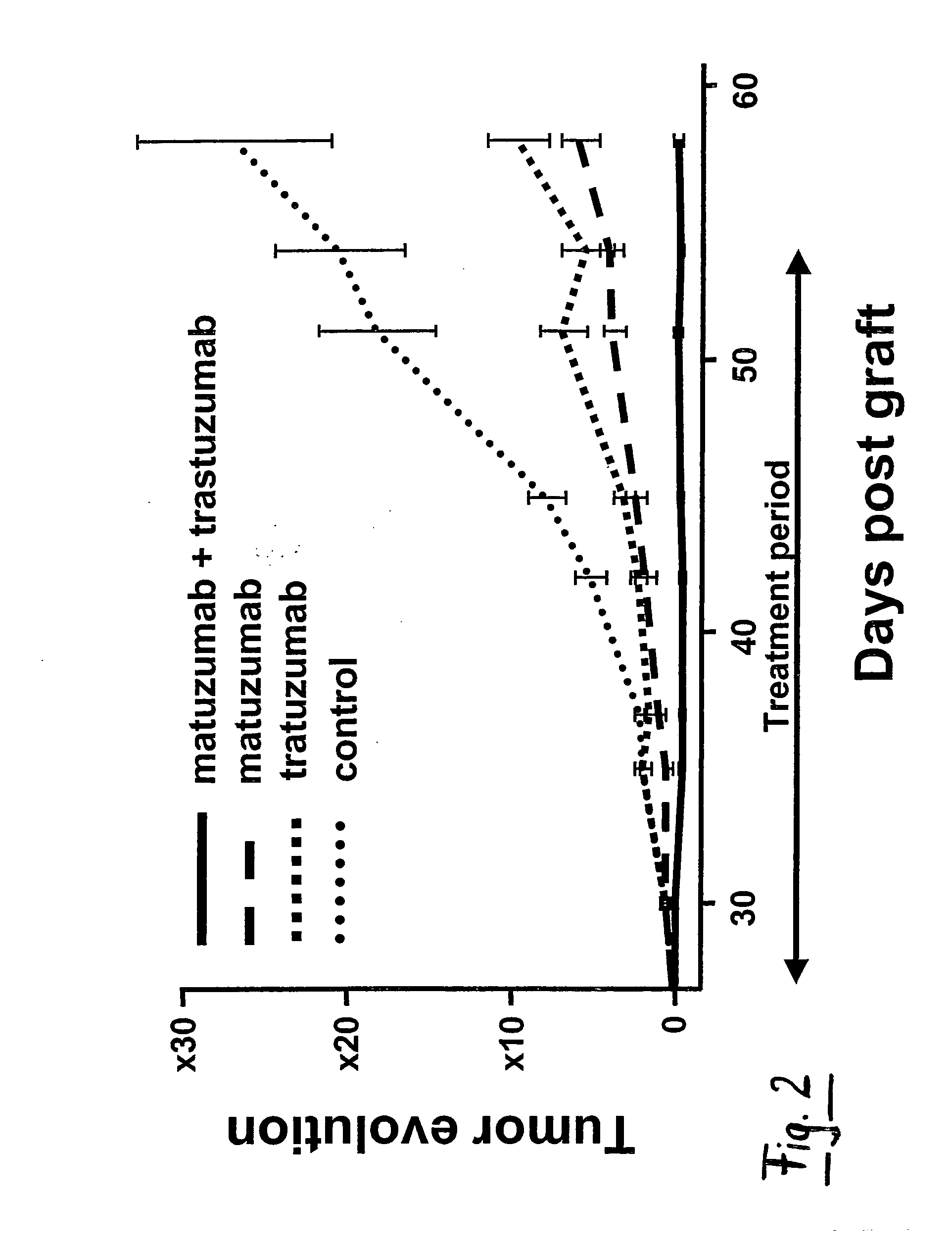
In Vivo Tumor Growth Inhibition Study
[0133]All in vivo experiments were performed in compliance with the French guidelines for experimental animal studies (Agreement No. B34-172-27). Nude mice, 6-8-week-old female athymic NMRI mice and BALB / c athymic mice were purchased from Janvier, Le Genest (St Isle, France) and Charles Rivers Laboratories (L'Arbresle, France), respectively. BxPC-3 (3.5×106), MiaPaCa-2 (5×106) and SK-OV-3 (5×106) cells were injected subcutaneously (s.c.) in to the right flank of athymic NMRI (BxPC-3 model) and BALB / c (MiaPaCa-2 and SK-OV-3) nude mice. MiaPaCa-2 cells were suspending in 50% culture medium and 50% Matrigel (BD biosciences, Le Pont De claix, France). Tumor-bearing mice were randomized in the different treatment groups when the tumors reached an approximate volume indicated in each experiment. For the BxPC-3 model, effects of antibody treatments were studied on small tumor (experiment S) and on large tumor (experiment L). The mice were treated by int...
example 2
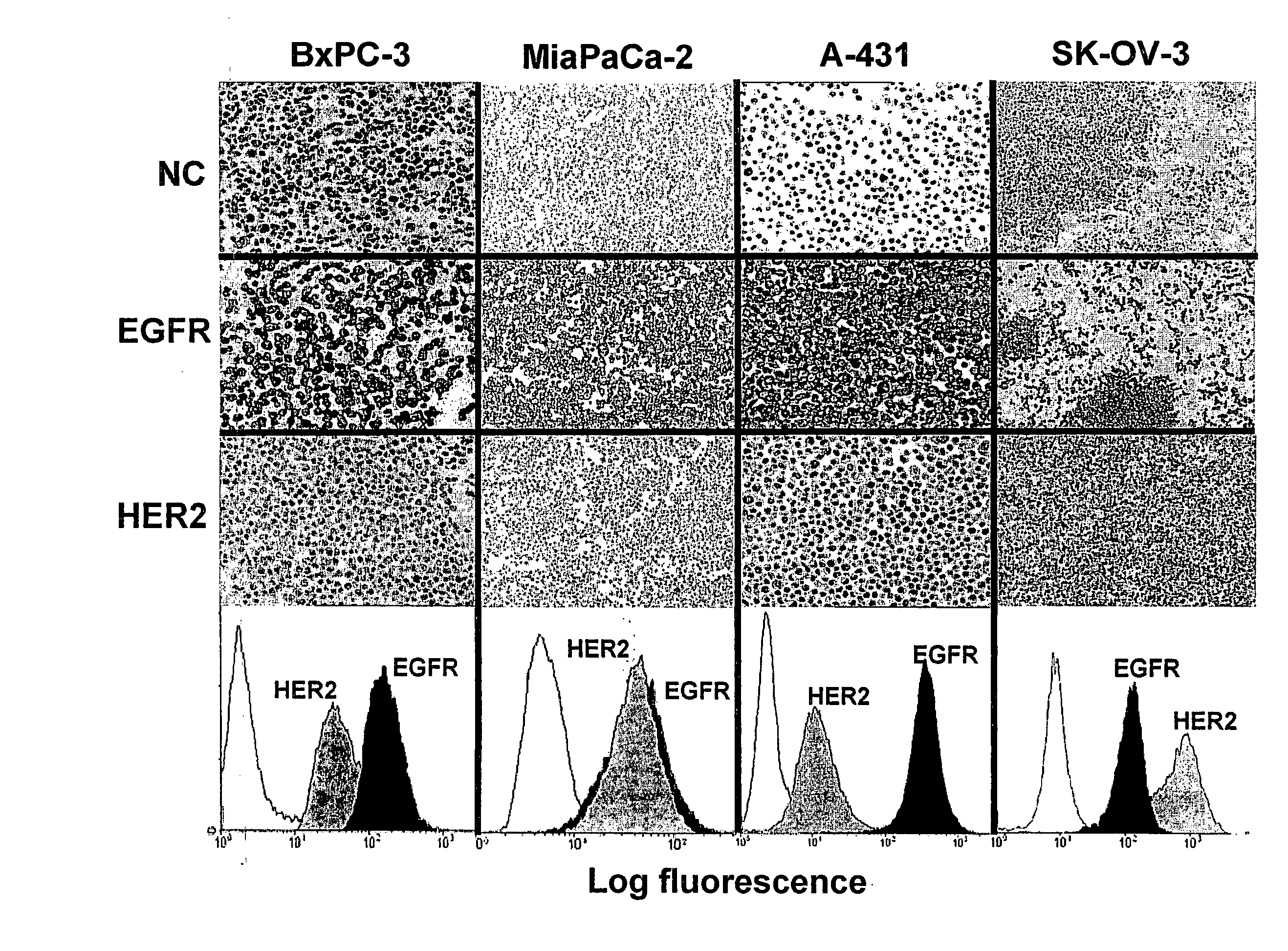
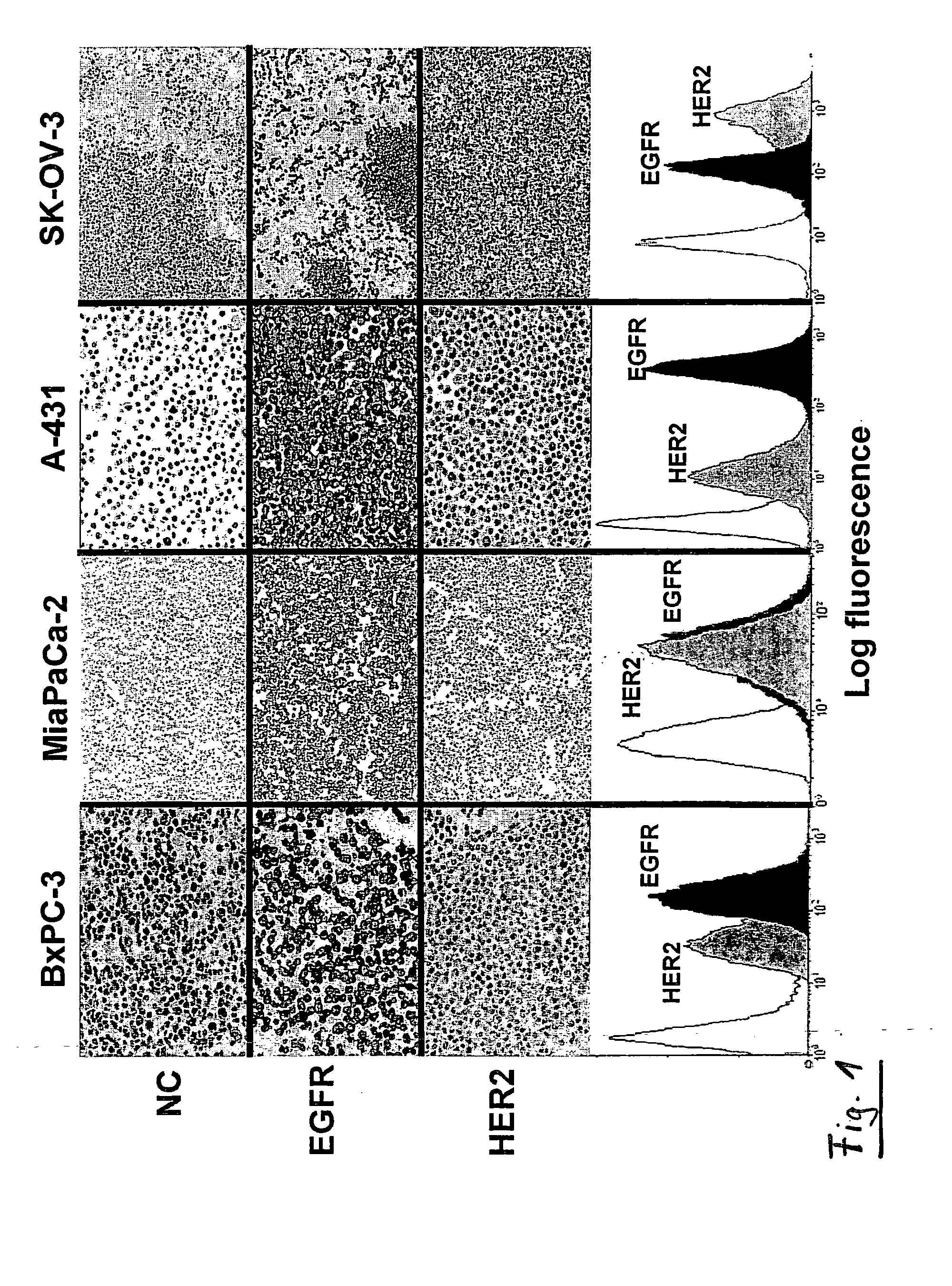
Immunocytochemical Analyses
[0134]Expression of receptors was analyzed in paraffin-embedded cells fixed in AFA (alcohol formol acetic acid). Analysis of EGFR expression was performed by using the EGFR pharmDx kit (DakoCytomation, Carpinteria, Calif., USA) according to the manufacturer's recommendations. Diaminobenzidine (Dakocytomation) was used as the chromogen, and the sections were lightly counterstained with hematoxylin. The primary antibody used for the detection of HER2 was a rabbit polyclonal antibody (Dakocytomation).
example 3
Immunoblotting Analysis
[0135]BxPC-3 and MiaPaCa-2 cells, plated at 106 cells for 24 h in Petri dishes were starved for two days in a medium without growth factors (SM medium) and treated with 10 Ng / ml of trastuzumab, matuzumab or both antibodies at a fixed 1:1 ratio (or controls without antibody). After a 48-h incubation, cells were incubated for 10 min in the SM medium with or without 100 ng / ml of EGF, washed twice, and lysed with buffer (CliniSciences SA, Montrouge France)-1-containing 100 NM PMSF, 100 mM sodium fluorure, 1 mM sodium orthovanate, and one complete protease inhibitor mixture tablet (Sigma, St Louis, Mo.). After electrophoresis on 8% SDS-PAGE under non-reducing conditions, the proteins were transferred to a polyvinylidene difluoride membranes (Millipore Co., Bedford, Mass.) which were saturated in PBS containing 0.1% Tween 20 and 5% nonfat dry milk and then incubated with the antibodies against the phosphorylated forms of EGFR and HER2, obtained from Cell Signaling T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com