Anti-PD-1 monoclonal antibody and application thereof
A monoclonal antibody, PD-1 technology, applied in the fields of application, antibody, anti-tumor drugs, etc., can solve the problems of ineffective activation of the immune system and immune escape of tumor cells, and achieve the recovery of lymphocyte activity, high binding rate, and specificity good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Animal immunization
[0028] The recombinant human PD-1 protein (dissolved in PBS buffer, which has been preliminarily identified, not the focus of this patent and will not be expanded) prepared in the laboratory in the early stage is mixed with Quick Antibody in an equal volume, and injected intramuscularly to 6-8 weeks old In BAL b / c mice, 50μg / 200μl / mice. The immunization process was carried out according to Quick Antibody instructions. A total of 3-5 immunizations were carried out. After each immunization for a period of time, the orbital blood (50 μl) of the immunized mice was collected by capillary blood sampling. The indirect ELISA was used to determine the serum antibody efficacy of the mice after serial dilution of the serum with the blocking solution. price. The mouse with the highest specific antibody titer in serum was selected for cell fusion. The final test results showed that the serum titer of the immunized mice could reach more than 1:100,0...
Embodiment 2
[0029] Example 2 Preparation of hybridoma cell lines
[0030] 1. Cell fusion
[0031] Cell fusion was performed using the polyethylene glycol method. The specific operations are as follows:
[0032] 1) One week before fusion, the sp2 / 0-Ag14 myeloma cells were recovered, and 8-azaguanine was used to adjust the cell state. Two days before cell fusion, sp2 / 0-Ag14 was expanded and cultured, and the state was observed to make it close to the logarithmic growth phase one day before fusion, and the medium was changed the day before fusion;
[0033] 2) Generally, when the serum titer of immunized mice reaches 100,000, the fusion can be achieved. Select the mouse with the highest PD-1-specific antibody titer in the serum, pulse immunization 3-4 days before fusion, and inject PD-1 protein into the abdominal cavity or tail vein;
[0034] 3) Preparation of feeder layer cells (mouse peritoneal macrophages): feeder layer cells were laid on the day before fusion, and older ICR mice (e.g....
Embodiment 3
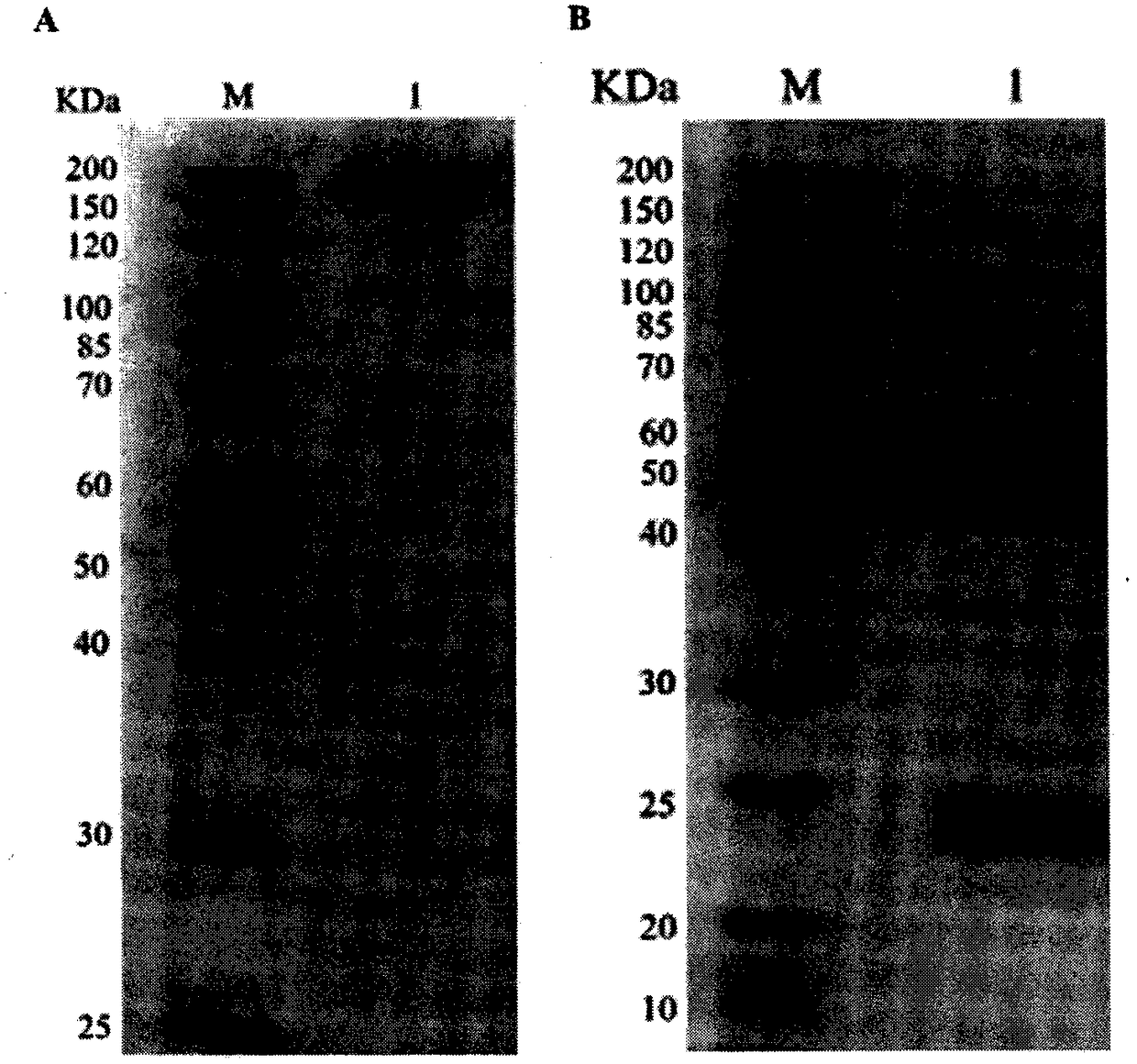
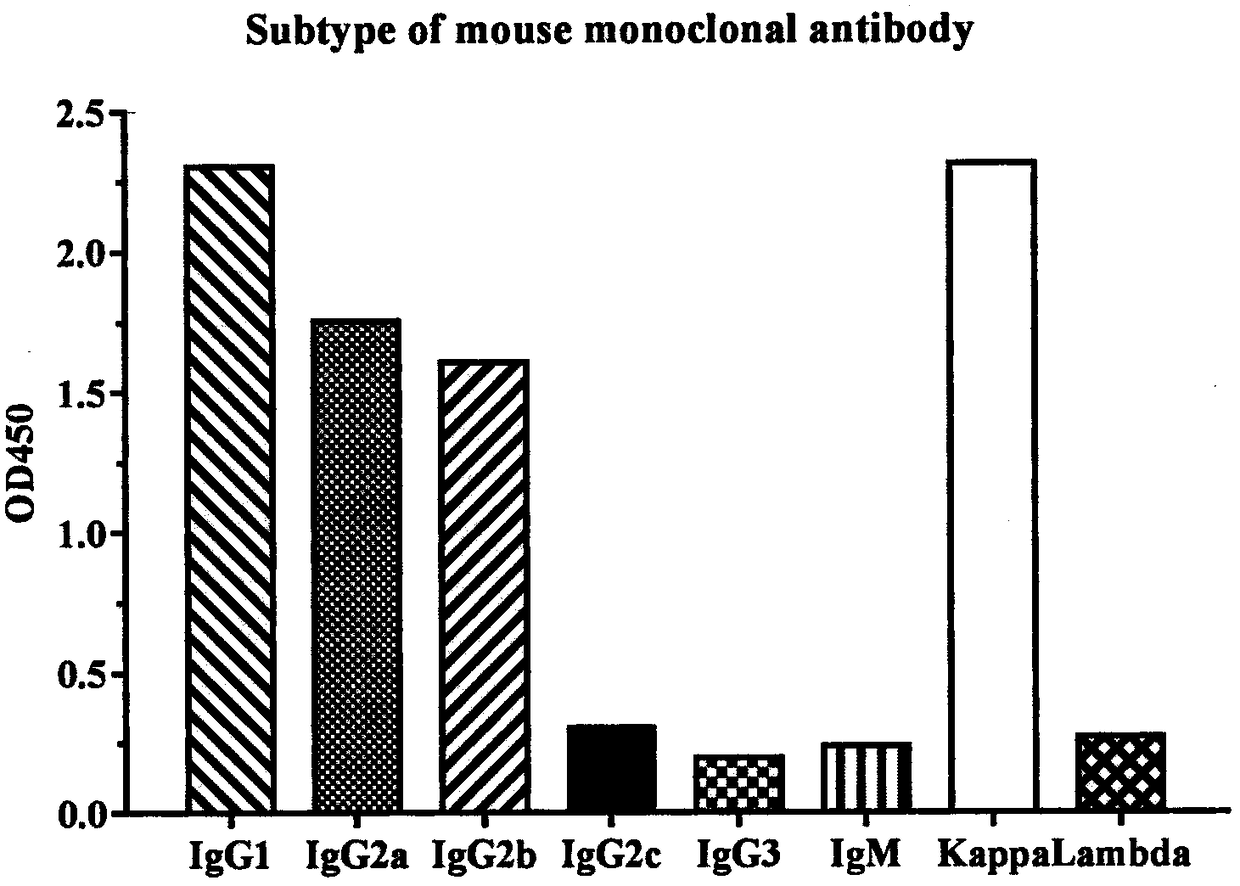
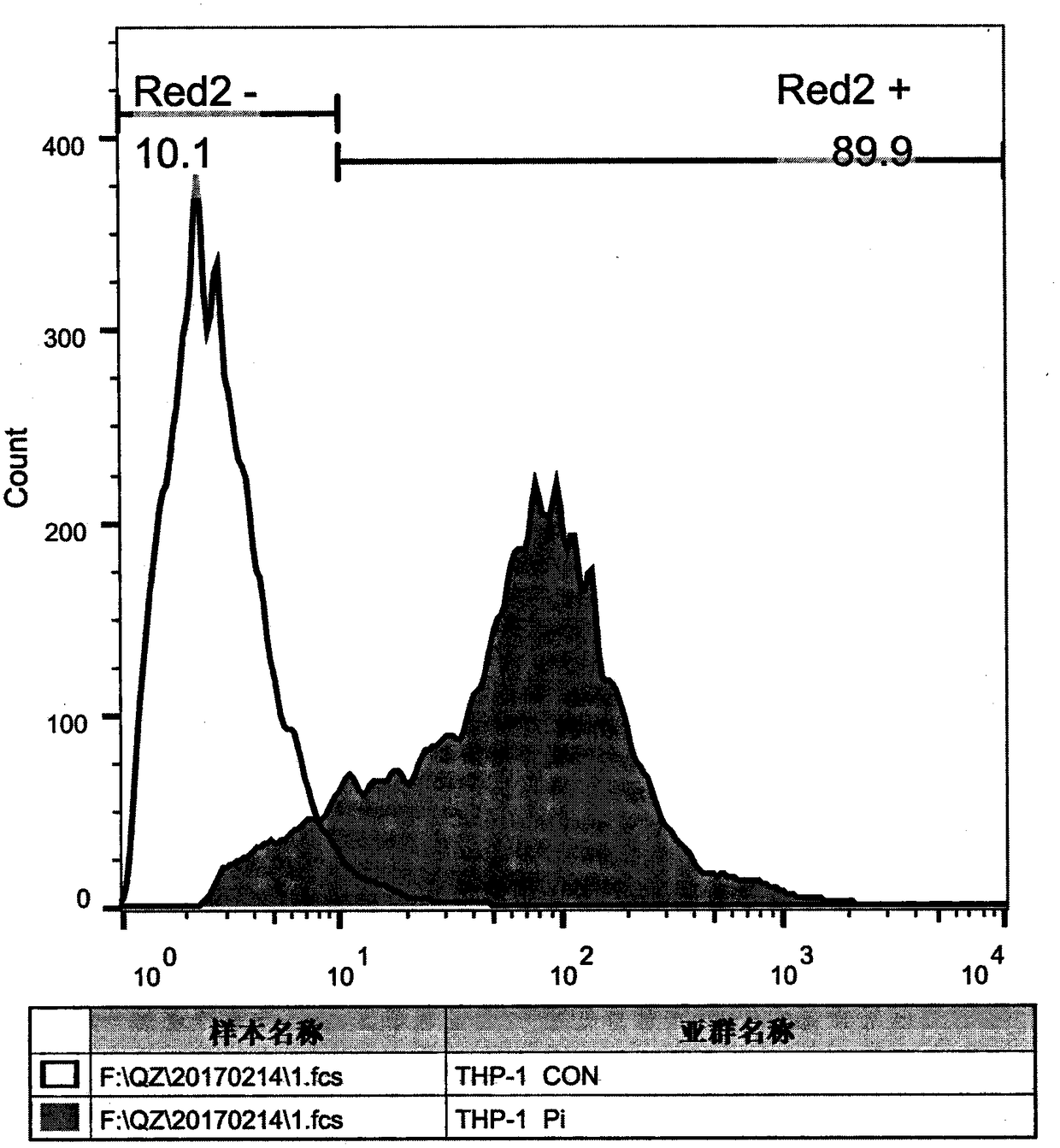
[0041] Example 3 Preparation and purification of anti-PD-1 monoclonal antibody
[0042] 1. Ascites Collection
[0043] Take the intraperitoneal injection of 500 μl of paraffin oil (SIGMA) into 8-10 week old female Bal b / c mice. One week later, intraperitoneal injection of 1 × 10 6 well-growing hybridoma cells. About 7-10 days after the hybridoma cells were inoculated, the abdomen of the mice was obviously bulging. At this time, the ascites was collected and centrifuged at 4°C at 12,000 rpm for 5 min to remove cell debris, and the supernatant was collected for subsequent purification.
[0044] 2. Antibody Purification
[0045] Antibody purification was performed using ProteinA / G column packing (Abmart). Specific operation: Ascites supernatant should be diluted with BindingBuffer to a certain volume and filtered and sterilized before loading. After loading, wash with Binding Buffer to remove non-specifically bound impurities, and finally elute, add 5-10ml Elution Buffer to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com