Method to induce and expand therapeutic alloantigen-specific human regulatory T cells in large-scale
An allogeneic antigen, allogeneic technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problem of lack of human antigen-specific Treg effective means, and achieve the effect of preventing rejection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
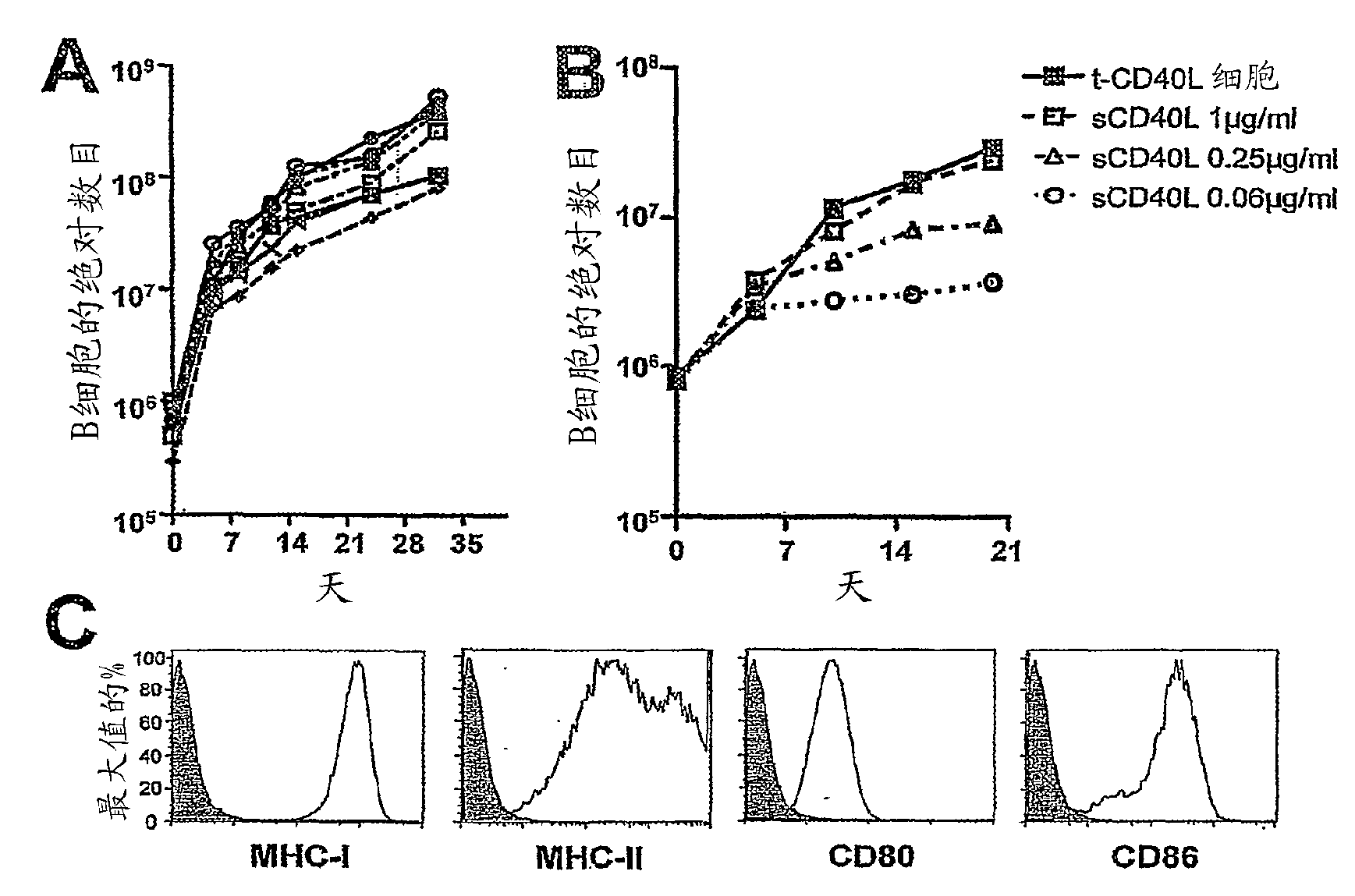
[0070] Example 1: CD40-activated B cells expanded by incubation with CD40-ligand transfected cells or soluble hexameric CD40-ligand express high levels of MHC and co-stimulatory molecules
[0071] as previously reported 27 , can be expanded from circulating B cells contained in PBMCs by treating NIH3T3 (t-CD40-L) cells transfected with CD40-ligand (CD40-L), IL-4, and low concentrations of cyclosporin A. Increases untransformed CD40-activated B cells. At day 8, CD19 + CD3 - The purity of B cells was at least 83% and over 95% at day 12. At 28-32 days in culture, more than 99% of cells are CD19 + CD3 - B cells. To evaluate the rate of expansion of B cells, we monitored CD19 production from 5.0 ml of peripheral blood from 8 unselected healthy adult donors + CD3 - Absolute number of cells. We found that after 32 days in culture, 8.1-54.3 x 10 7 CD40-activated B cells ( figure 1 A). We next determined whether soluble hexameric CD40-ligand (sCD40-L) could replace t...
Embodiment 2
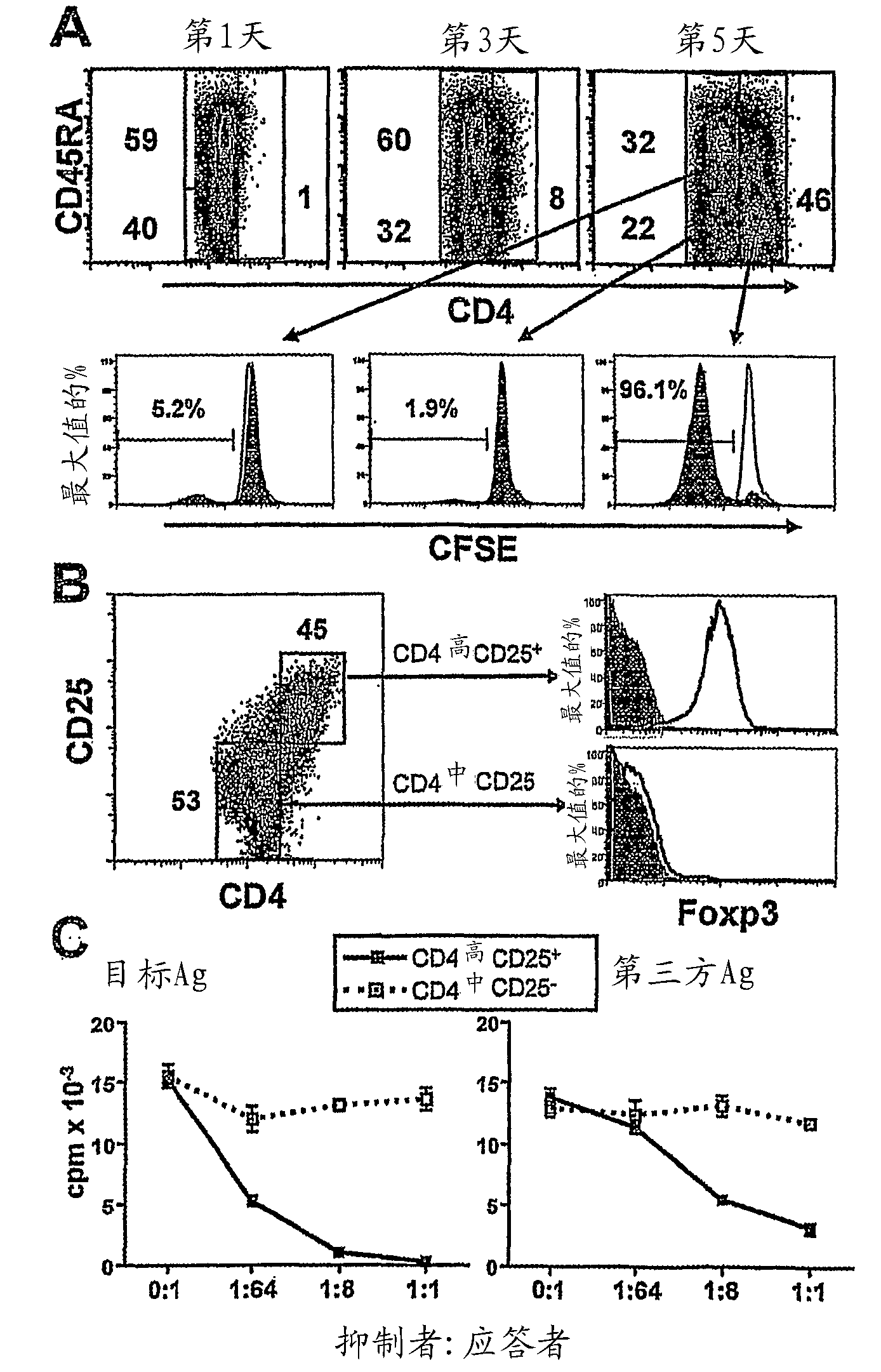
[0072] Example 2: Human alloreactive CD4 induced by CD40-activated B cells 高 cells are Treg
[0073] To determine whether allogeneic CD40-activated B cells could be converted from CD4 + CD25 - T cell induction of Treg, purified circulating CD4 stimulated with allogeneic CD40-activated B cells + CD25 - T cells (purity >99%) for 7 days. Surprisingly, after 5 days of allogeneic stimulation, a new subset of cells with significantly upregulated levels of CD4 surface expression was induced, and these CD4 高 Most of the cells lost CD45RA expression ( figure 2 A), and CD45RO expression was obtained (data not shown). In addition, these CD4 高 Most of the cells also lost CFSE staining, while CD4 中 Cells still maintain their CFSE content ( figure 2 A), showing the induction of CD4 高 The cells are proliferating alloreactive cells. These putative alloreactive CD4 高 Cells express CD25 and Foxp3, while CD4 中 Cells do not express these two Treg markers ( figure 2 B). Togethe...
Embodiment 3
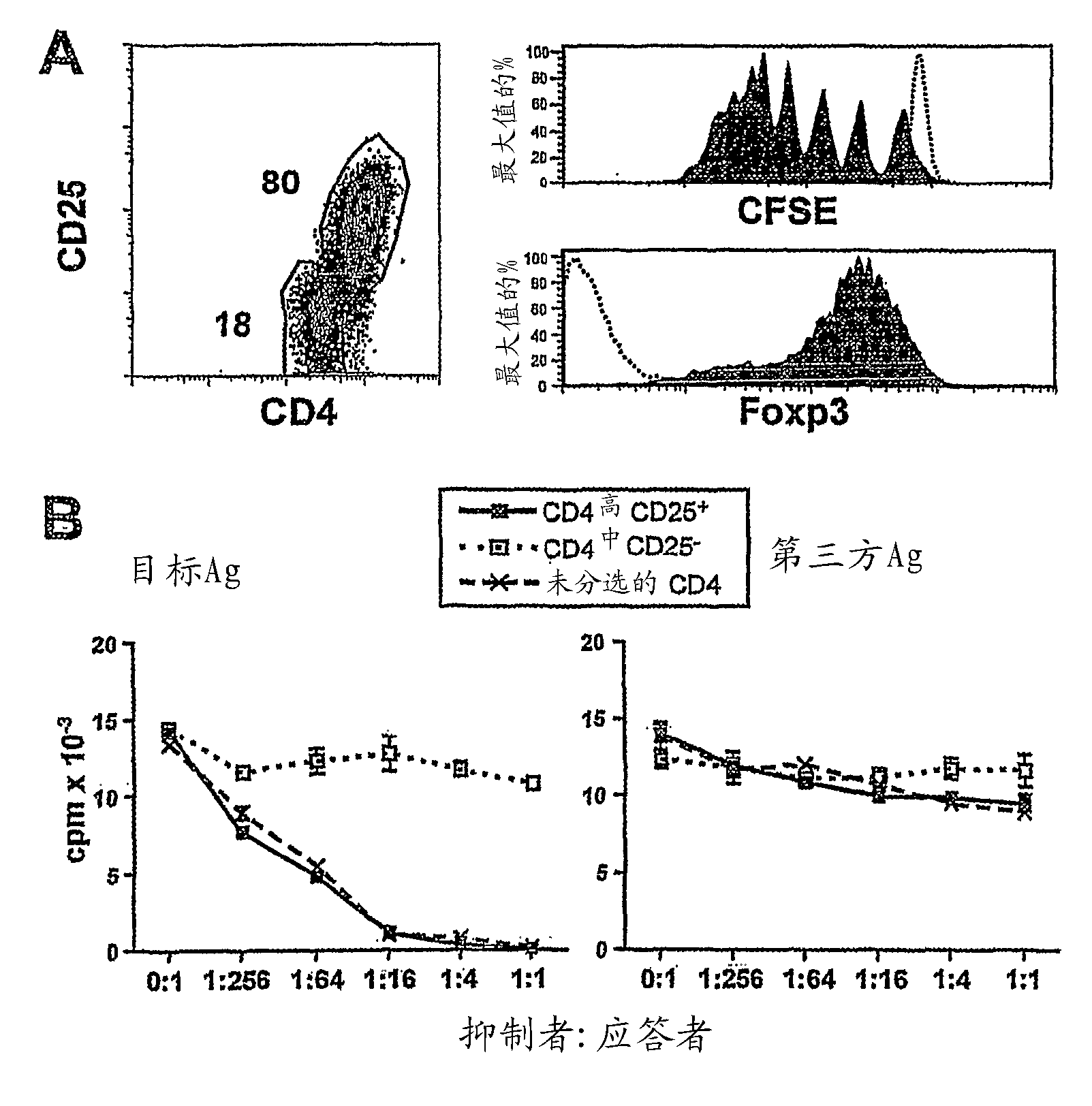
[0075] Example 3: CD40-activated B cells can be derived from naive CD4 + CD25 - alloantigen-specific CD4 高 CD25 + Tregs
[0076] We next determined whether, by co-culture with allogeneic CD40-activated B cells, + CD25 - cells (CD4 + CD45RA + CD45RO - CD25 + ) produces alloantigen-specific Tregs. As unisolated CD4 + CD25 - In the case of T cells, naive CD4 cells expanded by co-culture with CD40-activated B cells ± CD25 - Cells also acquired CD4 after 7 days in culture 高 , CD25 + and Foxp3 + Phenotype ( image 3 A). In addition, these CD4 高 CD25 + Foxp3 + Treg underwent 7-8 cell divisions during allogeneic stimulation for 7 days ( image 3 A). Instead, CD4 中 Cells neither divide nor express CD25 and Foxp3 ( image 3 A).
[0077] We further examined the induction of CD4 from naive precursors 高 CD25 + Treg suppressive capacity and alloantigen specificity. These CD4 高 CD25 + Treg significantly suppressed the original target alloantigen-induced p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com