Therapeutic Anti-cd40 ligand antibodies
An antibody and antigen technology, applied in the direction of antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, allergic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0073] All embodiments of the isolated antibodies or antigen-binding fragments thereof bind CD40L and inhibit or block the binding of CD40L to CD40. As used herein, "blocking the binding of CD40L and CD40" and "blocking the interaction between CD40L and CD40" are used interchangeably. Inhibiting or blocking binding can be direct or indirect. In general, the antibody or antigen-binding fragment thereof will compete specifically with CD40 for the same binding site on CD40L, or through steric positioning caused by binding of the antibody or antigen-binding fragment thereof near the CD40-binding site of CD40L. Block to physically interfere with the binding of CD40L to CD40. In other cases, the effect is indirect, eg, the antibody or antigen-binding fragment thereof causes an allosteric change in the conformation of CD40L, thereby inhibiting or eliminating its binding to CD40.
[0074]One embodiment is an isolated antibody that binds CD40L and comprises a light chain and a heavy ...
Embodiment 1
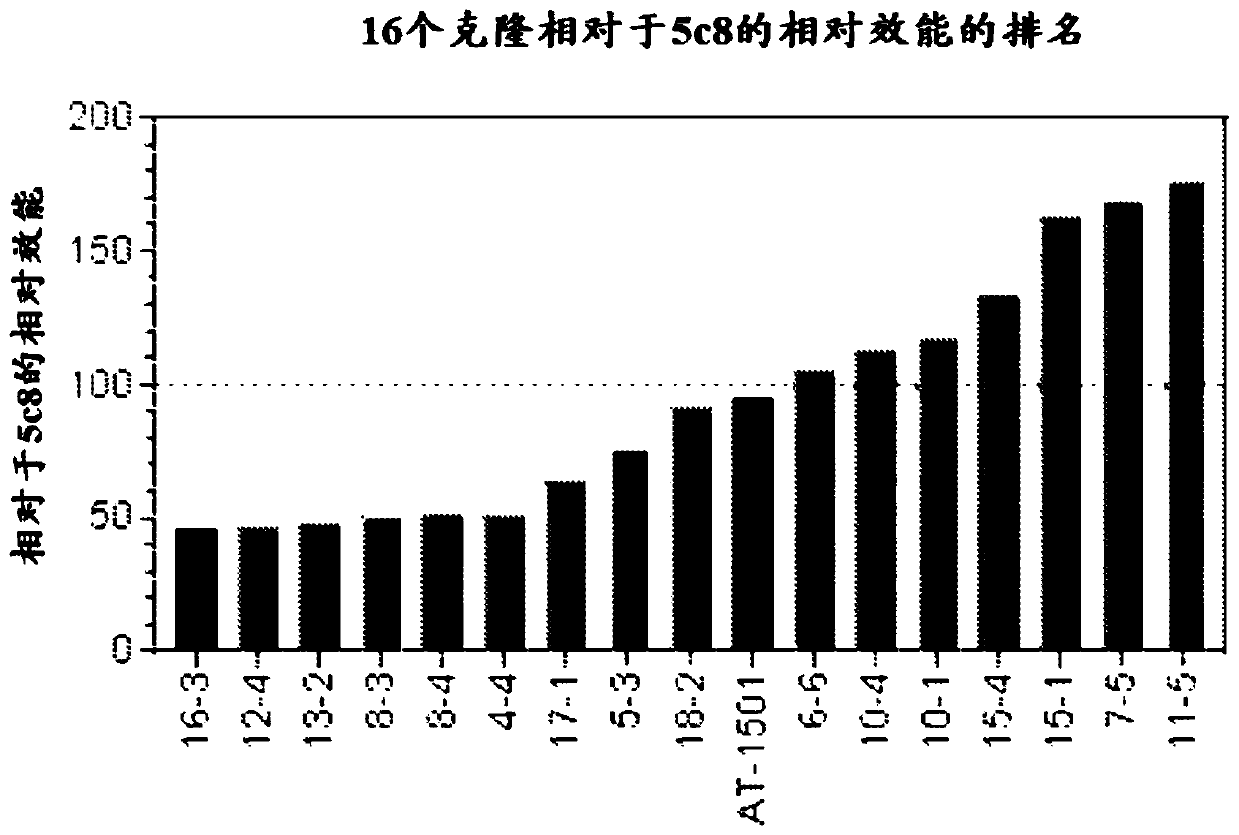
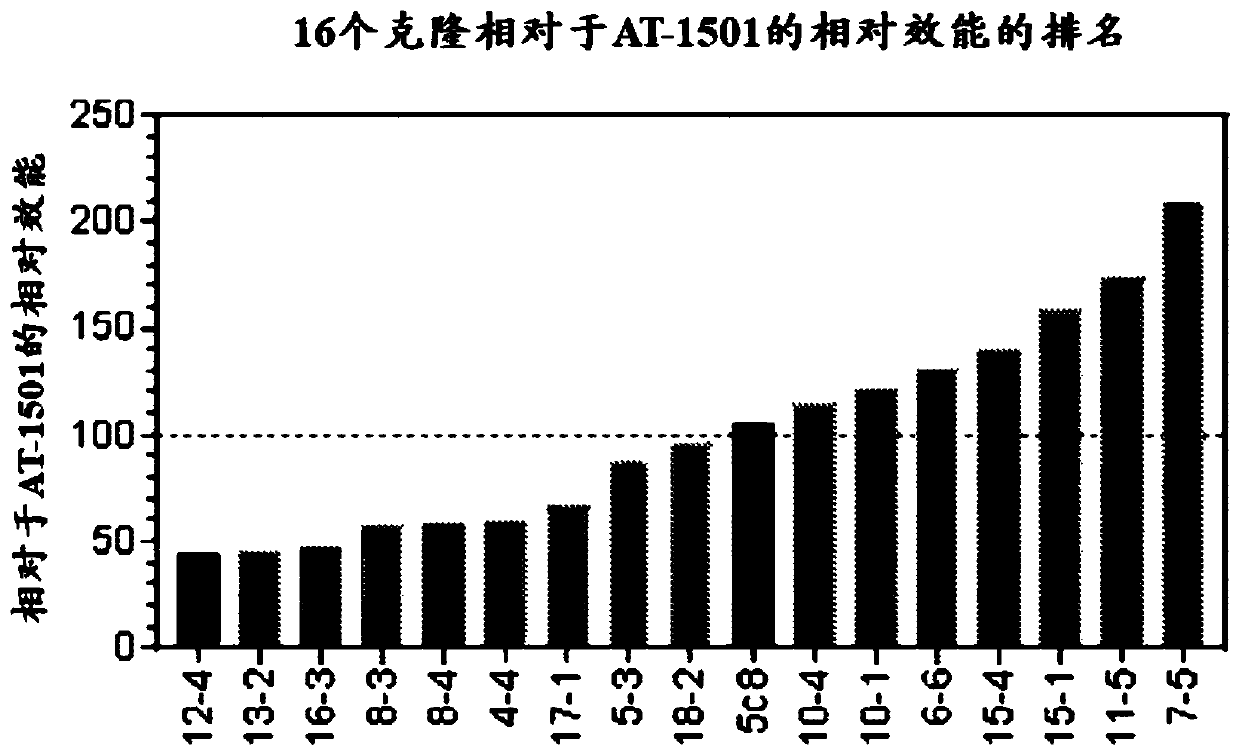
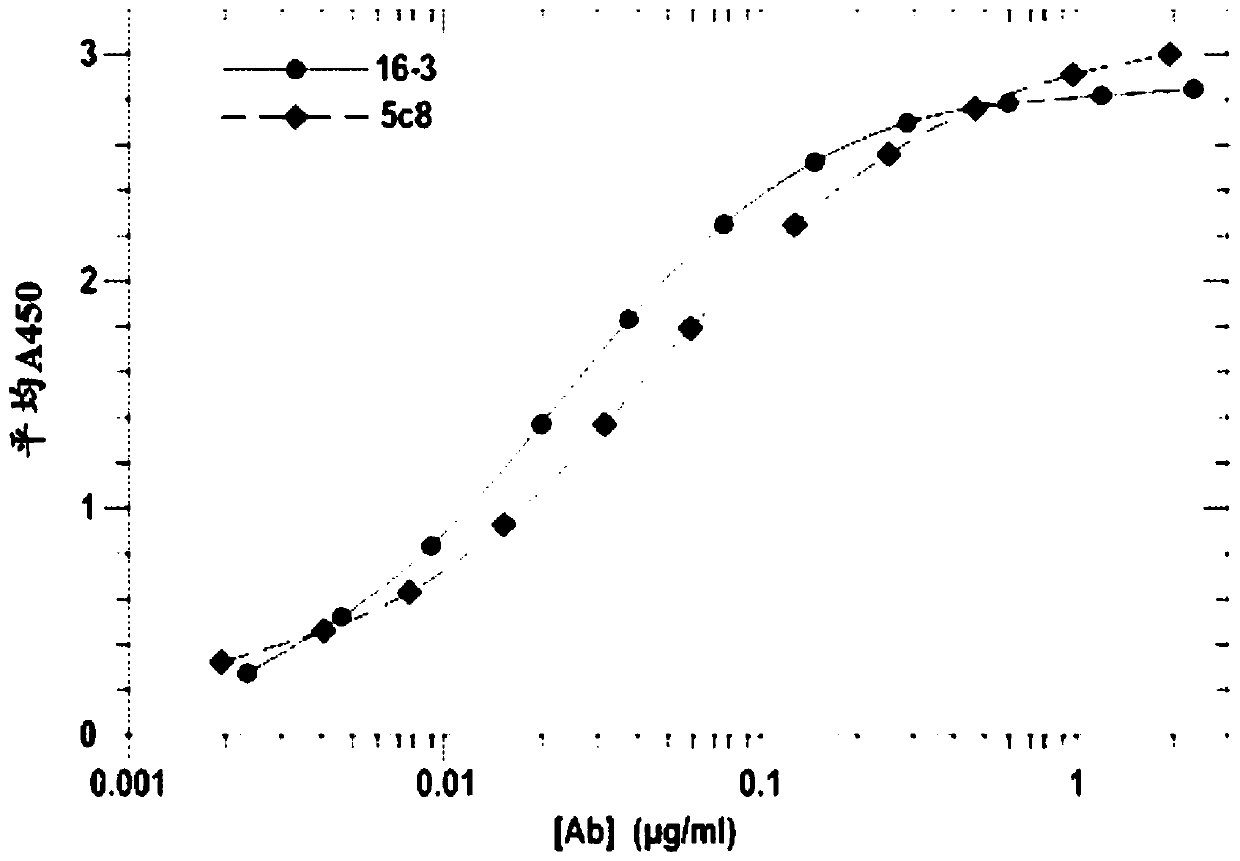
[0152] Example 1 CD40L binding assay
[0153] To compare CD40L binding of antibodies from all 16 clones relative to 5c8 or AT-1501, binding assays were performed using 2 clones, with 5c8 and AT-1501 run on the same 96-well assay plate. A three-part sandwich ELISA assay was used to determine the level of binding of the disclosed antibodies compared to reference antibodies 5c8-19 and AT1501. A 96-well polystyrene plate was coated with recombinant human CD40L in PBS (BioLegend cat# 591706) using 2ug / ml, and 50ul / well was added to a Costar 96-well half-area high binding assay plate (Corning 3690) and Incubate overnight at 4 °C. Plates were blocked with (1X) PBS / 1.0% BSA (140ul / well) for 1 hour at room temperature to prevent background binding. Add 5C8 or AT1501 (starting from 2ug / ml, serial 2-fold dilution) (50ul / well) for binding curve and incubate at room temperature for 1 hour. Plates were washed and incubated with 1:10,000 dilution of HRP-(Fab2) donkey anti-human IgG antibo...
Embodiment 2
[0161] Example 2 Binding activity to human FcγRI, FcγRIIa, FcγRIIIa and FcγRIIIb
[0162] Sixteen VH / VL antibody clones were constructed using IgGl Fc (SEQ ID NO: 21 ) with two mutations, P238S and N297G. These antibody clones were assayed for Fc effector function against binding to human FcyRI, FcyRIIa and FcyRIIIa.
[0163] Anti-CD40L antibody (abatacept included as negative control) was diluted to 2ug / ml in (1X) PBS, and 50ul / well was added to Costar 96-well half-area high binding assay plate (Corning 3690) for 4 Incubate overnight at °C. Plates were blocked with (1X) PBS / 1.0% BSA (140ul / well) for 1 hour at room temperature to prevent background binding. Recombinant human FcγRI, FcγIIa, FcγIIIa and FcγIIIb (serial 2-fold dilutions starting from 5ug / ml) (50ul / well) for binding curves were added and incubated at room temperature for 1 hour. Plates were washed and incubated with 2ug / ml mouse anti-human CD16 (anti-FcRIII), CD32 (anti-FcRIIa) or CD64 (anti-FcRI) antibodies (e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com