Use of compound
A compound and drug technology, applied in the field of medical immunology, can solve problems such as body damage, achieve remarkable effects, avoid immune response, and suppress immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
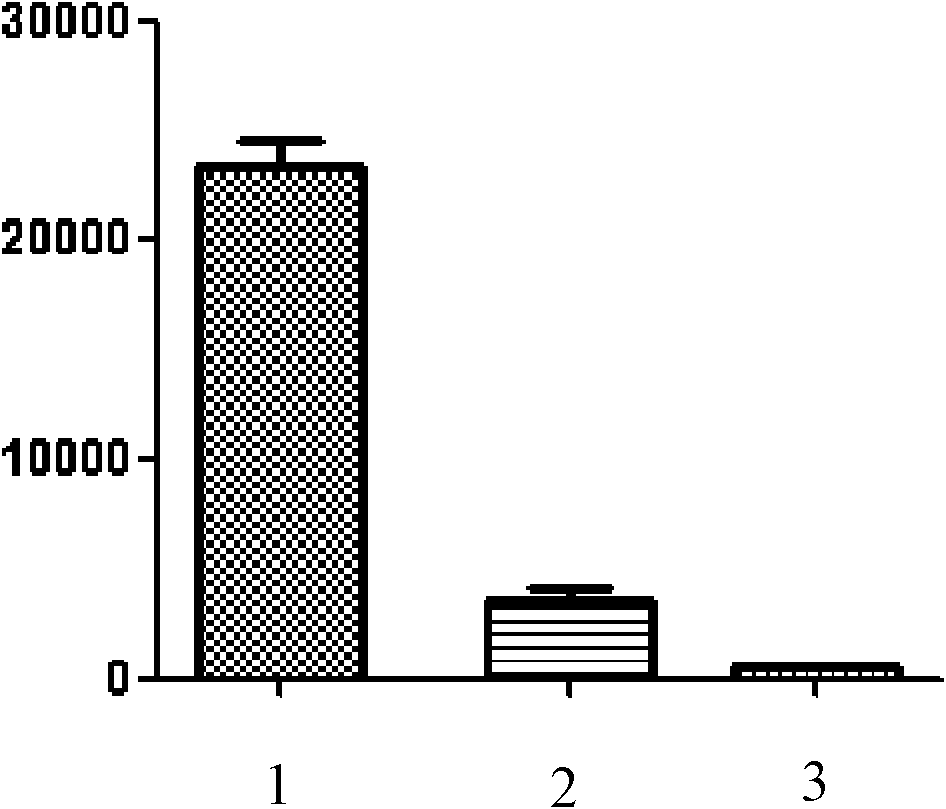
[0042] Example 1: Mouse T cell proliferation inhibition test
[0043] After aseptically separating spleen cells from mice and lysing red blood cells, add them to 96-well culture plates, 1-2×10 5 Cells / 100 μL / well. Add anti-CD3 (final concentration 1 μg / mL), anti-CD28 monoclonal antibody (final concentration 1 μg / mL), recombinant IL-2 (final concentration 10 Unit / mL) to stimulate in vitro, 100 μL / well, suppose to add 2-amino-7-( Hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-ethyl carboxylate was the test group, 3 wells / group; no 2-amino-7-(hydroxyimino)- Ethyl 4,5,6,7-tetrahydrobenzothiophene-3-carboxylate was used as the control group, 3 wells / group. Simultaneously, set as the blank group without the above-mentioned in vitro stimulation and without adding 2-amino-7-(hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-carboxylate ethyl ester, 3 hole / group.
[0044] Three groups of samples were placed at 37°C, 5% CO 2 Incubate for 66h under the condition, add 0.5-1μci to ea...
Embodiment 2
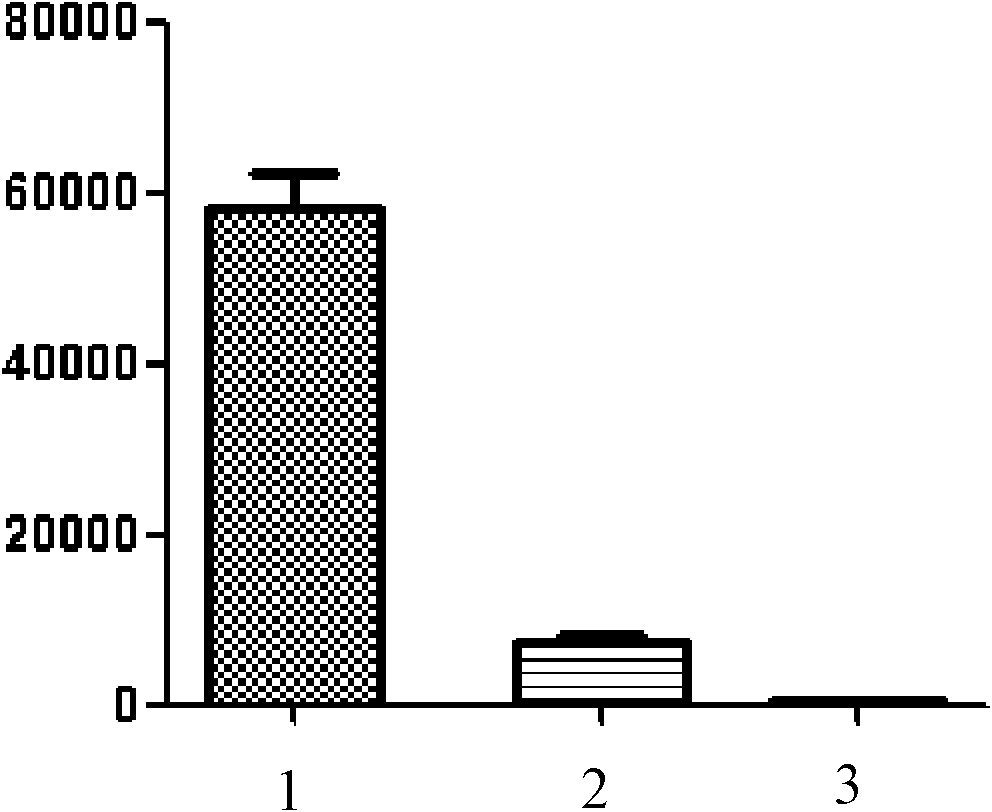
[0046] Embodiment 2: human T cell proliferation inhibition test
[0047] Under sterile conditions, isolate peripheral blood mononuclear cells (PBMC) from heparin anticoagulated blood, and adjust the number of cells to 1-2×10 with 10% FCS RPMI1640 after washing 6 / mE, added to 96-well culture plate, 1-2×10 5 Cells / 100 μL / well. Add T cell mitogen PHA, 100 μL / well, set 2-amino-7-(hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-carboxylic acid ethyl ester as the test group , 3 wells / group; without 2-amino-7-(hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-carboxylate ethyl ester as the control group, 3 wells / group. Simultaneously, set as the blank group without the above-mentioned in vitro stimulation and without adding 2-amino-7-(hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-carboxylate ethyl ester, 3 hole / group.
[0048] Three groups of samples were placed at 37°C, 5% CO 2Incubate for 66h under the condition, add 0.5-1μci to each well 3 H-TdR 50μL, continue to culture...
Embodiment 3
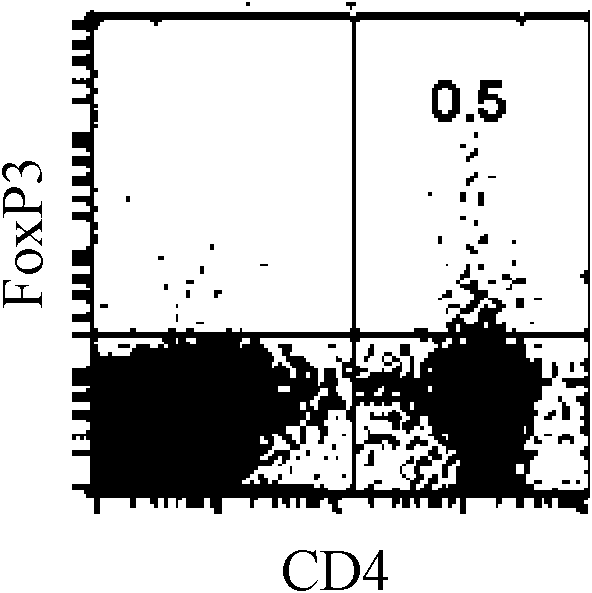
[0050] Example 3: Mouse FoxP3 + CD4 + Regulatory T cell induction assay
[0051] FoxP3 + CD4 + Regulatory T cells are a type of T cells with immunosuppressive effects. This type of cells participates in the regulation of immune response / immune tolerance in an "active" manner, not only participates in the regulation of autoimmune tolerance, but also plays an important role in transplantation immunity. In order to prove whether ethyl 2-amino-7-(hydroxyimino)-4,5,6,7-tetrahydrobenzothiophene-3-carboxylate can induce naive T cells to differentiate into FoxP3 + CD4 + Regulatory T cells, after the present invention isolates mouse splenocytes and stimulates them in vitro, FoxP3 is detected by flow cytometry (FACS) + CD4 + Differentiation of regulatory T cells. Specific steps are as follows:
[0052] According to the method of Example 1, it is divided into a control group, a test group and a blank group, and then cultured in a 24-well plate, 1-2 × 10 6 / mL, 2mL / well. At 37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com