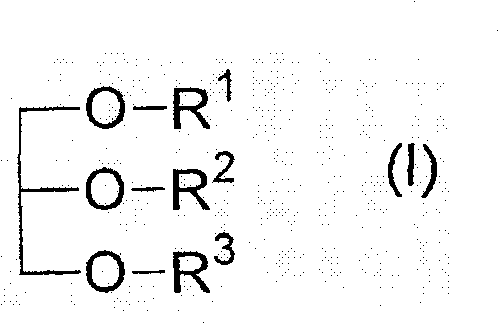Fat emulsion for artificially feeding seriously ill intensive care patients
A technology of fat emulsion and fatty acid residues, applied in fat emulsion, prevention and treatment of critically ill polyneuropathy) and critically ill myopathy) pharmaceutical preparations, fat emulsion in artificial feeding of sepsis intensive care patients The application field can solve the problems such as unknown secondary complications
Inactive Publication Date: 2011-10-12
B BRAUN MEDICAL
View PDF8 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, little is known about effective nutritional approaches to treat sepsis and secondary complications of intensive care treatment, such as CIP and / or CIM
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0054]
[0055] 1) For the fraction of medium-chain triglycerides (caprylic / capric triglycerides), a commercially available mixture was used (Miglyol 812, Sasol Germany GmbH, Witten, Germany).
[0056] 2) As structured lipids, glycerol enptylated in the sn-1 and sn-3 positions of triglycerides and eicosapentaenoic acid (EPA; C20:5 Ω -3) Composition of triglycerides (A).
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
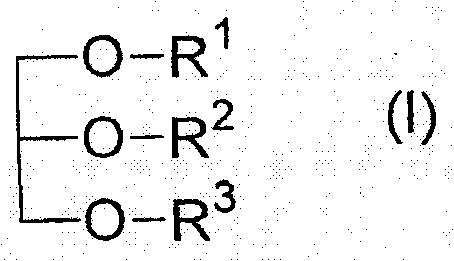
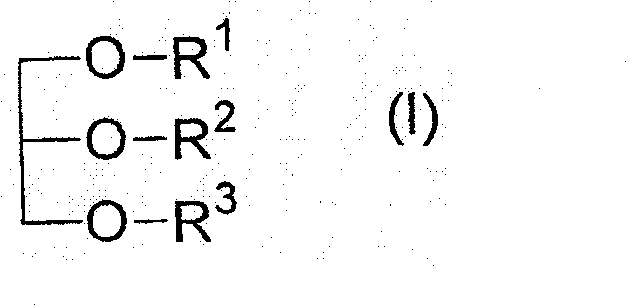
The present invention relates to a pharmaceutical preparation for the prophylaxis and treatment of critical illness polyneuropathy (CIP) and critical illness myopathy (CIM). The invention further relates to an isotonic fat emulsion comprising at least one triglyceride that comprises at least one fatty acid group having an odd number of carbon atoms, wherein the fatty acid group comprises a carbon chain having 5 to 15 carbon atoms.
Description
technical field [0001] The present invention relates to pharmaceutical preparations for the prevention and treatment of critical illness polyneuropathy (CIP) and critical illness myopathy (CIM). Furthermore, the present invention relates to an isotonic fat emulsion comprising at least one triglyceride comprising at least one fatty acid residue having an odd number of carbon atoms, wherein said fatty acid residue comprises carbon chain. Furthermore, the invention relates to the use of said isotonic fat emulsion as a dietary product, and the invention also relates to the use of said pharmaceutical preparation / isotonic fat emulsion in the context of parenteral nutrition or as a component of a dietary product, in particular the fat emulsion in Application of artificial feeding in intensive care patients with sepsis. Background technique [0002] The survival time of extremely ill intensive care patients can be significantly prolonged due to developments in intensive care medic...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/23A23L1/30A61P3/02
CPCA61K31/225A23L33/12A61K9/0029A61K9/107A61K9/1075A61K31/22A61K31/23A23V2002/00A61P21/00A61P25/00A61P25/02A61P3/02A61P31/04A61P43/00A61K2300/00A61K31/20
Inventor 迈克尔·博尔
Owner B BRAUN MEDICAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com