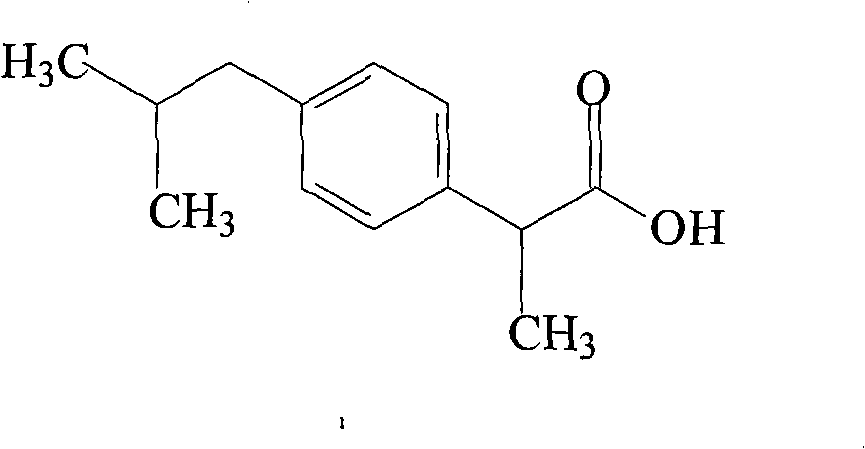
Method for preparing ibuprofen injection
An injection, alkaline technology, applied in the direction of pharmaceutical formulations, drug delivery, non-central analgesics, etc., can solve the problems of slow absorption, gastrointestinal irritation, low solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
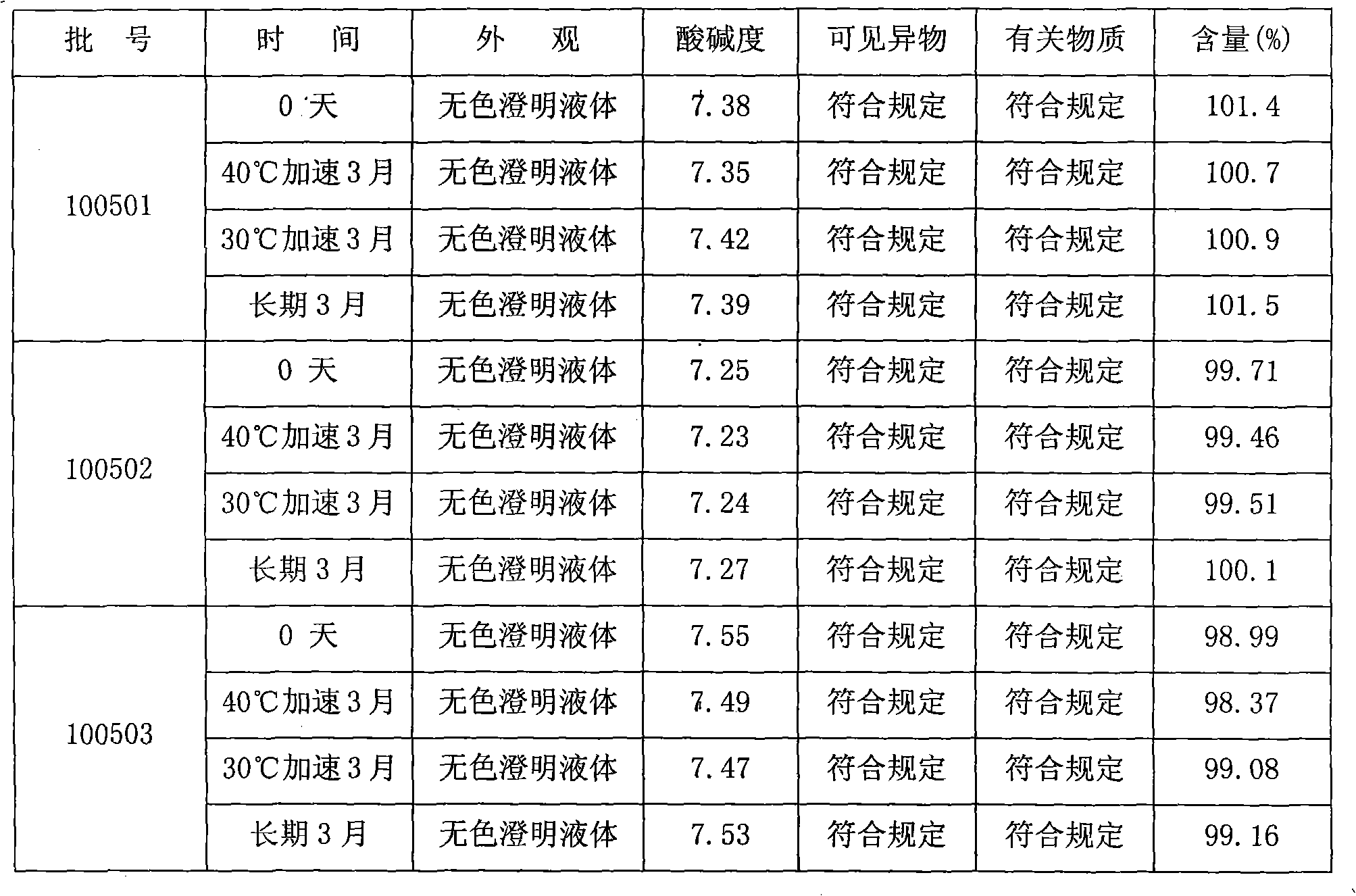
[0014] Embodiment 1: Ibuprofen injection, 400mg specification (batch number: 100501)
[0015] Ibuprofen 400g
[0016] Arginine 312g
[0017] Sodium bicarbonate 7g
[0018] Edetate Disodium 2g
[0019] Add water for injection 4000ml
[0020]
[0021] 1000 pieces
[0022] The sodium bicarbonate and arginine that take prescription quantity are dissolved in 2800ml (70%) water for injection and dissolve completely, slowly add ibuprofen under stirring condition until fully dissolving, add prescription quantity disodium edetate and dissolve, add water to Stir well enough, add 2g (0.05%) of activated carbon, stir for 15-30min, decarbonize, filter through 0.45μm, 0.22μm microporous membrane; pack into brown ampoules according to 4ml, sterilize at 121℃ for 10 minutes to make injection liquid.
Embodiment 2
[0023] Embodiment 2: ibuprofen injection, 400mg specification (batch number: 100502)
[0024] Ibuprofen 400g
[0025] Lysine 260g
[0026] Disodium hydrogen phosphate dodecahydrate 156g
[0027] Sodium dihydrogen phosphate dihydrate 7.5g
[0028] Sodium metabisulfite 4g
[0029] Add water for injection 4000ml
[0030]
[0031] 1000 pieces
[0032] The disodium hydrogen phosphate, sodium dihydrogen phosphate and lysine that take prescription quantity are dissolved in 3200ml (80%) water for injection and dissolve completely, slowly add ibuprofen under stirring condition until completely dissolving, add prescription quantity sodium pyrosulfite to dissolve , add water to a sufficient amount and stir evenly, add 2g (0.05%) of activated carbon, stir for 15-30 minutes, decarbonize, filter through 0.45μm, 0.22μm microporous membranes; pack into brown ampoules according to 4ml, and sterilize at 121°C for 10 minutes Made into ...
Embodiment 3
[0033] Embodiment 3: ibuprofen injection, 800mg specification (batch number: 100503)
[0034] Ibuprofen 800g
[0035] Diethanolamine 360g
[0036] Borax 80g
[0037] Add water for injection 8000ml
[0038]
[0039] 1000 pieces
[0040] The diethanolamine and borax that take prescription quantity are dissolved in 6000ml (75%) water for injection and dissolve completely, under stirring condition, slowly add ibuprofen until fully dissolved, add water to sufficient amount and stir evenly, add 2g (0.05%) of activated carbon , stirred for 15-30 minutes, decarbonized, filtered through 0.45 μm and 0.22 μm microporous membranes; divided into brown ampoules according to 8ml, and sterilized at 121°C for 10 minutes to make injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com