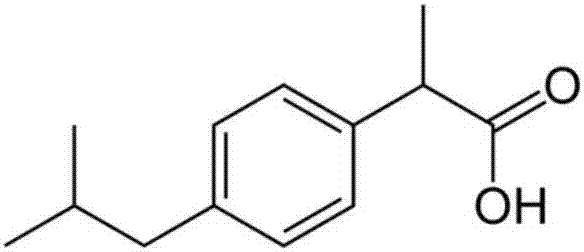
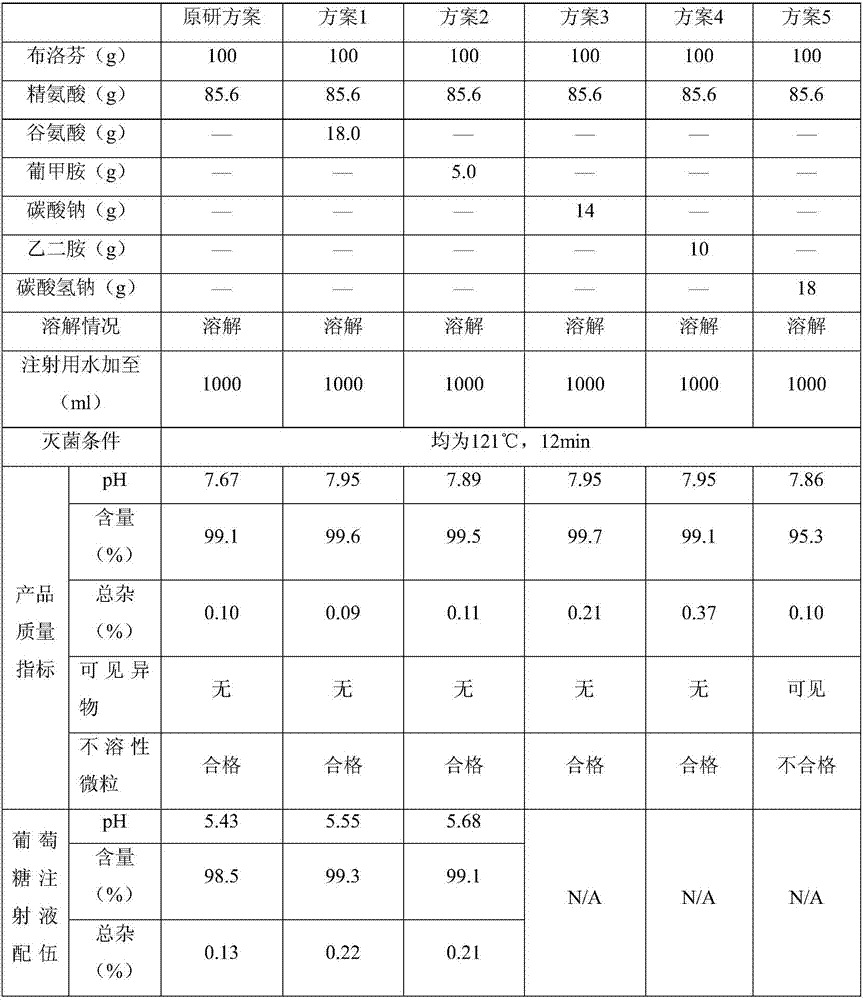
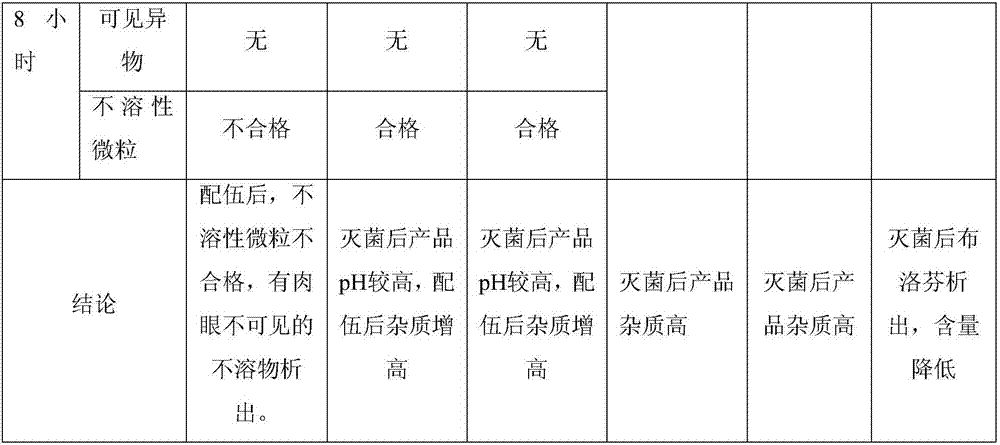
Ibuprofen injection for intravenous administration
An injection and intravenous technology, which is applied in the field of ibuprofen injection and its preparation, can solve problems such as high pH of the final product, pitting in ampoules, and pain at the injection site, so as to improve compatibility stability, reduce clinical risks, and reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Prescription (1000 formulation units):
[0055] ibuprofen
[0056] Preparation process: Add water for injection with a total volume of about 7200ml into the container, add 684.8g of arginine and 40g of poloxamer 188, stir until completely dissolved, add 800g of ibuprofen, stir until completely dissolved, add 4g of needle Use activated carbon, keep warm and stir for 30 minutes, decarburize by filtering with a 0.45μm filter element, add water for injection to 8000ml, and decarburize through a 0.20μm filter element, fill it into an ampoule, and sterilize it at 121°C for 12 minutes to obtain the product.
Embodiment 2
[0058] Prescription (1000 formulation units):
[0059] ibuprofen
[0060] Preparation process: Add water for injection with a total volume of about 7200ml into the container, add 684.8g arginine and 80g poloxamer 188, stir until completely dissolved, add 800g of ibuprofen, stir until completely dissolved, add 4g of needle Use activated carbon, keep warm and stir for 20 minutes, decarburize by filtering with a 0.45 μm filter element, add water for injection to 8000 ml, decarbonize through a 0.20 μm filter element, fill and seal it into an ampoule, and sterilize it at 121°C for 12 minutes to obtain the product.
Embodiment 3
[0062] Prescription (1000 formulation units):
[0063] ibuprofen
[0064] Preparation process: Add water for injection with a total volume of about 7200ml into the container, add 684.8g arginine and 100g poloxamer 188, stir until completely dissolved, add 800g of ibuprofen, stir until completely dissolved, add 4g of needle Use activated carbon, keep warm and stir for 20 minutes, decarburize by filtering with a 0.45 μm filter element, add water for injection to 8000 ml, decarbonize through a 0.20 μm filter element, fill and seal it into an ampoule, and sterilize it at 121°C for 12 minutes to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com