Medicinal composition and preparation thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as kidney damage, frequent drug use to maintain therapeutic concentration, short biological half-life, etc., and achieve excellent quality results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
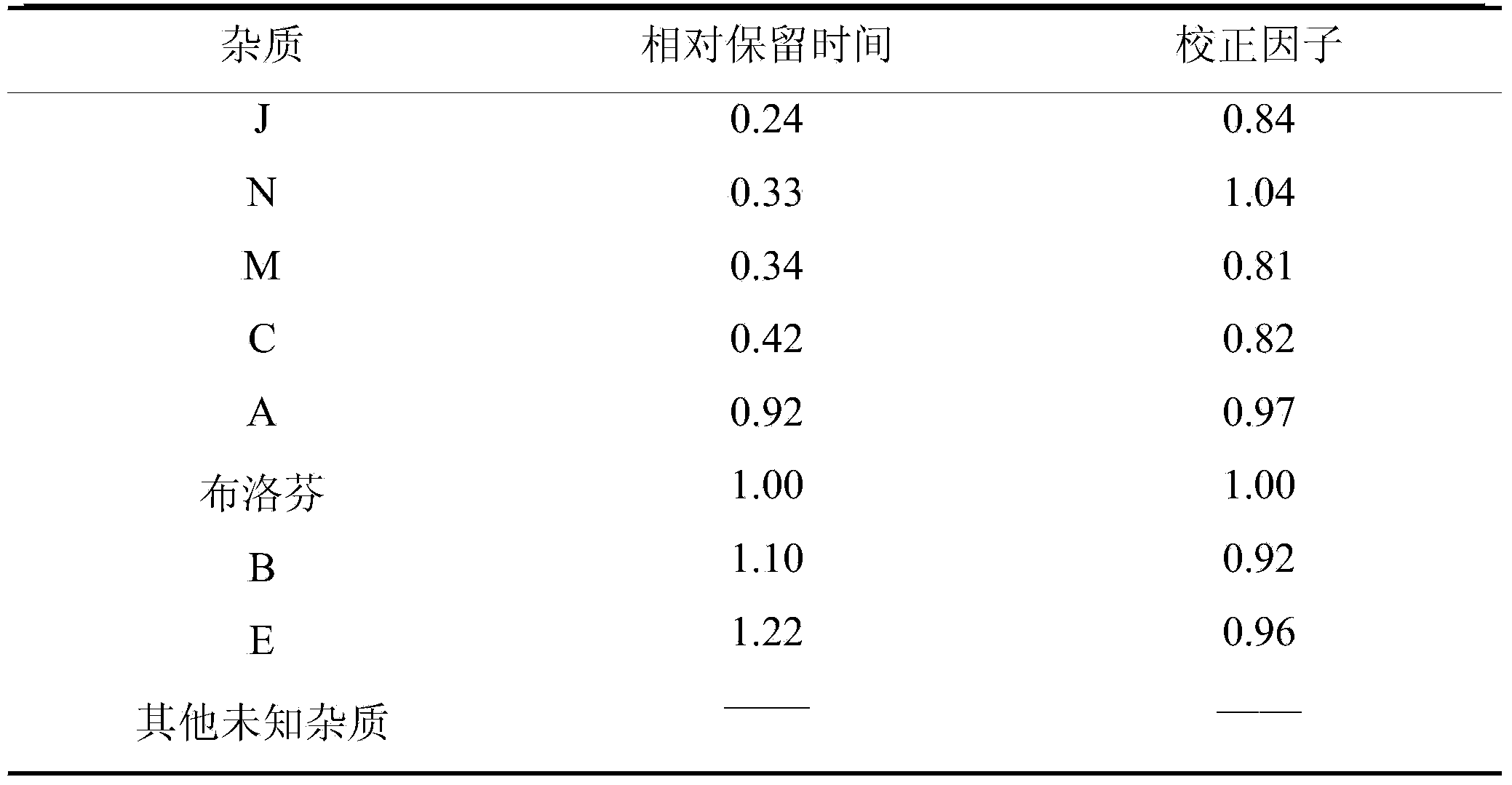
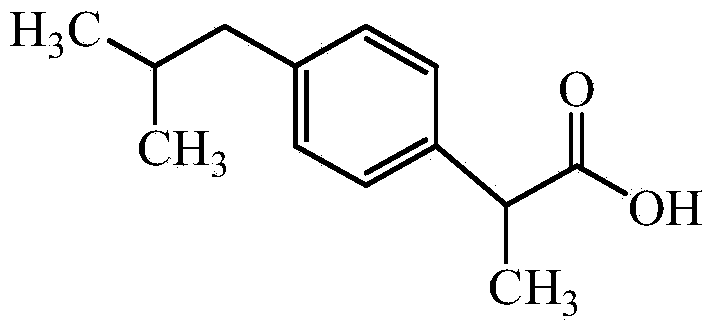
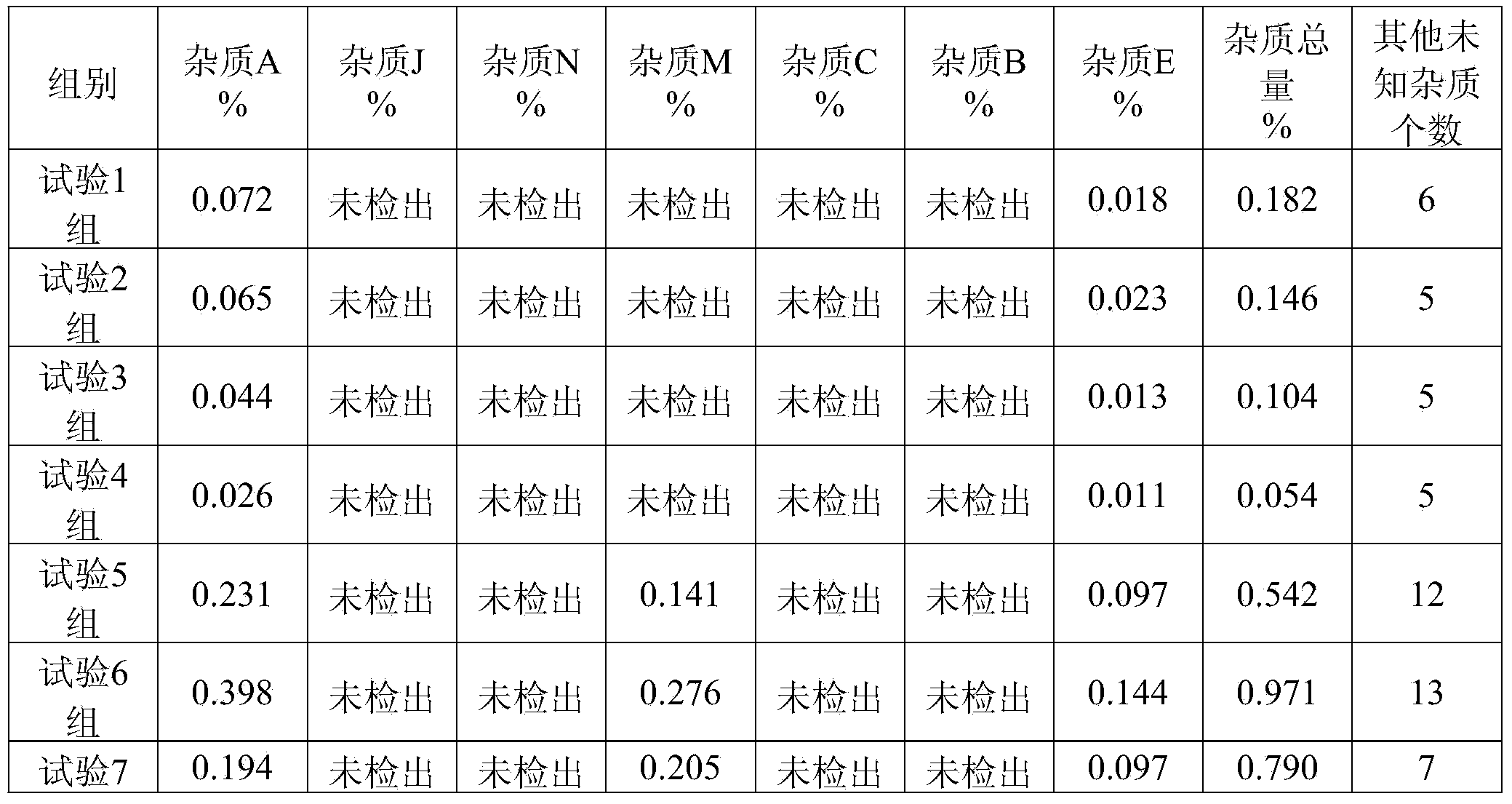
[0075] 100g of ibuprofen, 83g of arginine, water for injection, and a pH regulator of arginine and ethanolamine in a weight ratio of 1:0.2.
[0076] Take the temperature as 50°C,
[0077] Add 60% of the dosage of water for injection into a closed liquid mixing tank, and pass nitrogen for 15 minutes; weigh arginine and add it to water for injection at 30°C and keep stirring for 30 minutes; weigh ibuprofen and add it to water for injection at 30°C and keep stirring 30 minutes; adjust the pH value to 7.4 with a pH regulator, and stir for 30 minutes; add activated carbon for needles with a weight-to-volume ratio of 0.1%, stir for 30 minutes at 50°C, filter and decarbonize, and add water for injection to 100%; after 0.22 Filter with a microporous membrane of μm; use neutral ampoules to fill and seal under nitrogen protection; sterilize at 121.0°C for 15 minutes to obtain 1,000 bottles of water injection (4ml per bottle).
Embodiment 2
[0079] 100g of ibuprofen, 83g of arginine, water for injection, and a pH regulator of arginine and ethanolamine in a weight ratio of 1:0.2.
[0080] Take the temperature as 60°C,
[0081] Add 60% of the dosage of water for injection into a closed liquid mixing tank, and pass nitrogen for 15 minutes; weigh arginine and add it to water for injection at 30°C and keep stirring for 30 minutes; weigh ibuprofen and add it to water for injection at 30°C and keep stirring 30 minutes; adjust the pH value to 7.8 with a pH regulator, and stir for 30 minutes; add activated carbon for needles with a weight-to-volume ratio of 0.1%, stir for 30 minutes at 60°C, filter and decarbonize, and add water for injection to 100%; after 0.22 Filter with a microporous membrane of μm; use neutral ampoules to fill and seal under nitrogen protection; sterilize at 121.0°C for 15 minutes to obtain 1,000 bottles of water injection (4ml per bottle).
Embodiment 3
[0083] 100g of ibuprofen, 83g of arginine, water for injection, and a pH regulator of arginine and ethanolamine in a weight ratio of 1:0.2.
[0084] Take the temperature as 55°C,
[0085] Add 60% of the dosage of water for injection into a closed liquid mixing tank, and pass nitrogen for 15 minutes; weigh arginine and add it to water for injection at 30°C and keep stirring for 30 minutes; weigh ibuprofen and add it to water for injection at 30°C and keep stirring 30 minutes; adjust the pH value to 7.4 with a pH regulator, and stir for 30 minutes; add activated carbon for needles with a weight-to-volume ratio of 0.1%, stir for 30 minutes at 55°C, filter and decarbonize, and add water for injection to 100%; after 0.22 Filter with a microporous membrane of μm; use neutral ampoules to fill and seal under nitrogen protection; sterilize at 121.0°C for 15 minutes to obtain 1,000 bottles of water injection (4ml per bottle).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com