Emulsion injection containing dexibuprofen and preparation method of emulsion injection
A technology of Dexibuprofen and injectable milk, which is applied in the field of medicine and can solve the problems of poor water solubility of Dexibuprofen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A Dexibuprofen injection emulsion is characterized in that 150g of Dexibuprofen, 500g of soybean oil, 50g of phospholipid, 10g of oleic acid, 10g of poloxamer and 100g of glycerol are included in the injection emulsion.
[0054] Its preparation method is:
[0055] (1) Take glycerin, add water for injection, dissolve it, make glycerin aqueous solution, keep it at 50-60°C, use it as the water phase, and set aside;
[0056] (2) Weigh phospholipids, poloxamers and soybean oil, mix them, stir and dissolve them at 50-70°C, and use them as the oil phase;
[0057] (3) Take oleic acid and dextrobuprofen and add to the oil phase, stir, mix well, and set aside;
[0058] (4) Add (3) to (1) and mix while cutting, and high-speed shearing to form uniform colostrum;
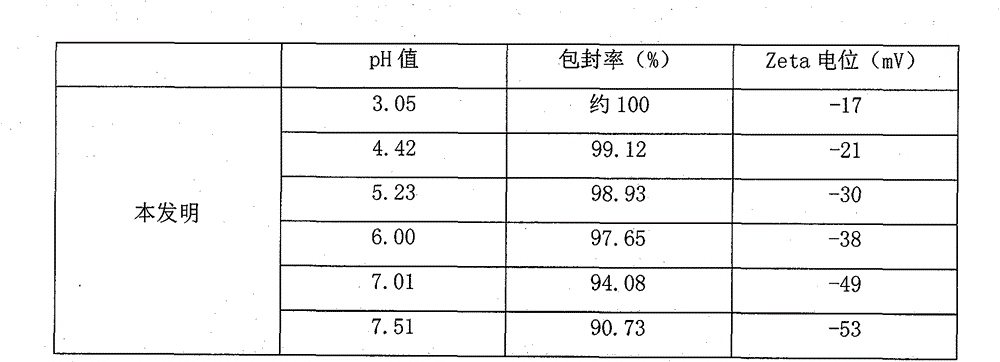
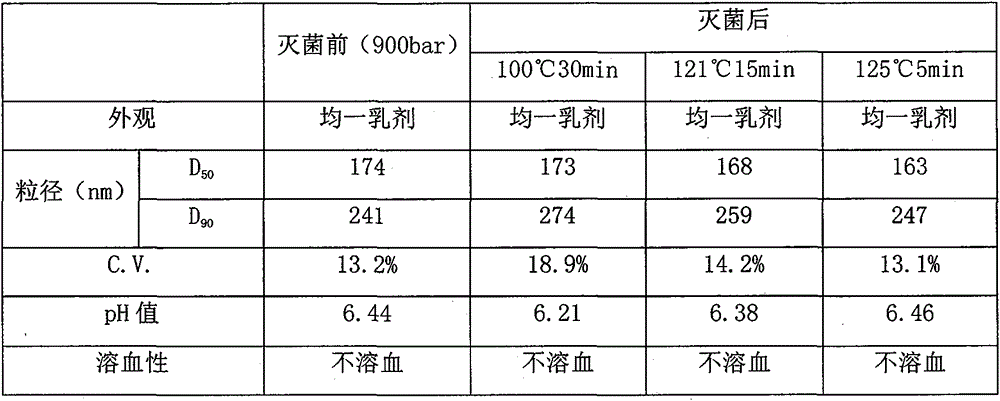
[0059](5) Dilute the obtained colostrum to 5000ml, and adjust the pH value to 4.0-9.0. After mixing evenly, pass through a homogenizer at 900bar for 6 times to obtain lipids with uniform particle size and an average par...
Embodiment 2
[0062] A Dexibuprofen injection emulsion is characterized in that 150g of Dexibuprofen, 1000g of soybean oil, 60g of phospholipid, 25g of oleic acid, 30g of poloxamer and 112.5g of glycerol are contained in the injection emulsion.
[0063] Its preparation method is:
[0064] (1) Take glycerin, add water for injection, dissolve it, make glycerin aqueous solution, keep it at 50-60°C, use it as the water phase, and set aside;
[0065] (2) Weigh phospholipids, poloxamers and soybean oil, mix them, stir and dissolve them at 50-70°C, and use them as the oil phase;
[0066] (3) Take oleic acid and dextrobuprofen and add to the oil phase, stir, mix well, and set aside;
[0067] (4) Add (1) to (3) and mix while shearing, and high-speed shearing to form uniform colostrum;
[0068] (5) Dilute the obtained colostrum to 5000ml, and adjust the pH value to 4.0-9.0. After mixing evenly, pass through a homogenizer at 900bar for 6 times to obtain lipids with uniform particle size and an avera...
Embodiment 3
[0071] A Dexibuprofen injection emulsion is characterized in that 250g of Dexibuprofen, 1000g of soybean oil, 60g of phospholipid, 25g of oleic acid, 30g of poloxamer and 112.5g of glycerol are included in the injection emulsion.
[0072] Its preparation method is:
[0073] (1) Take glycerin, add water for injection, dissolve it, make glycerin aqueous solution, keep it at 50-60°C, use it as the water phase, and set aside;
[0074] (2) Weigh phospholipids, poloxamers and soybean oil, mix them, stir and dissolve them at 50-70°C, and use them as the oil phase;
[0075] (3) Take oleic acid and dextrobuprofen and add to the oil phase, stir, mix well, and set aside;
[0076] (4) (1) and (3) are mixed, and the homogenizer is sheared at high speed to form uniform colostrum;
[0077] (5) Dilute the obtained colostrum to 5000ml, and adjust the pH value to 4.0-9.0. After mixing evenly, pass through a homogenizer at 900bar for 6 times to obtain lipids with uniform particle size and an a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com