Dexibuprofen enteric-coated and sustained-release tablet and preparation method thereof
A technology of dextro-ibuprofen and sustained-release tablets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc. and other problems, to achieve excellent sustained-release effect, eliminate irritation, and the effect of light and small tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
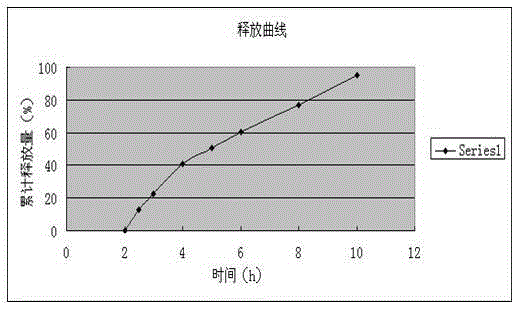
Examples
Embodiment 1
[0084] The preparation of embodiment 1 Dexibuprofen enteric-coated sustained-release tablets:
[0085] Tablet core prescription composition (g / 1000 tablets:
[0086] Dexibuprofen 300
[0087] Hydroxypropylmethylcellulose (HPMC)-K4M40
[0088] Hydroxypropyl Methyl Cellulose (HPMC)-K100LV20
[0089] Lactose 30
[0090] 75% ethanol solution Q.S
[0091] Micropowder silica gel 3
[0093] Enteric coating formula:
[0094] Acrylic resin (Eudragit® L30D-55) 160g
[0095] Triethyl citrate 4.8g
[0096] Glyceryl monostearate 2.4g
[0097] Tween-801.0g
[0098] 160g of water
[0099] 【Description of preparation process】
[0100] (1) Pass the prescription amount of Dexibuprofen through an 80-mesh sieve, and mix well with hypromellose (K4M), hypromellose (K100LV) and lactose;
[0101] (2) Add an appropriate amount of 75% ethanol to prepare soft materials, and pass through a 24-mesh sieve to obtain wet granules;
[0102] (3) Dry the wet g...
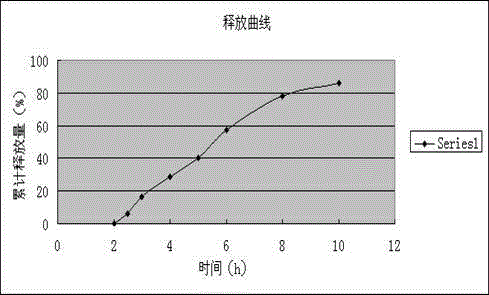
Embodiment 2
[0111] The preparation of embodiment 2 Dexibuprofen slow-release tablets
[0112] Tablet core prescription composition (g / 1000 tablets:
[0113] Dexibuprofen 300
[0114] Hydroxypropylmethylcellulose (HPMC)-K4M40
[0115] Hydroxypropyl Methyl Cellulose (HPMC)-K100LV20
[0116] Lactose 30
[0117] 75% ethanol solution Q.S
[0118] Micropowder silica gel 3
[0120] Enteric coating formula:
[0121] Acrylic resin (Eudragit® L30D-55) 160g
[0122] Triethyl citrate 4.8g
[0123] Glyceryl monostearate 2.4g
[0124] Tween-801.0g
[0125] 160g of water
[0126] 【Description of preparation process】
[0127] (1) Pass the prescription amount of Dexibuprofen through an 80-mesh sieve, and mix well with hypromellose (K4M), hypromellose (K100LV) and lactose;
[0128] (2) Add an appropriate amount of 75% ethanol to prepare soft materials, and pass through a 24-mesh sieve to obtain wet granules;
[0129] (3) Dry the wet granules in a blast dr...
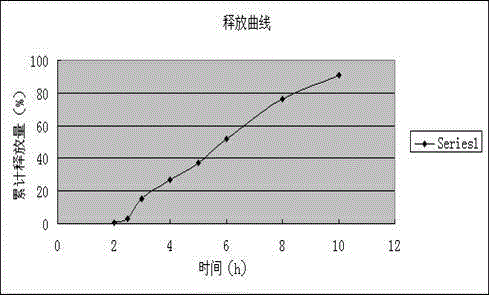
Embodiment 3
[0135] The preparation of embodiment 3 Dexibuprofen slow-release tablets
[0136] Tablet core prescription composition (g / 1000 tablets):
[0137] Dexibuprofen 300
[0138] Hydroxypropylmethylcellulose (HPMC)-K4M45
[0139] Hydroxypropyl Methyl Cellulose (HPMC)-K100LV15
[0140] 85% ethanol solution Q.S
[0141] Micropowder silica gel 3
[0143] Coating formula:
[0144] Acrylic resin (Eudragit® L30D-55) 160g
[0145] Triethyl citrate 4.8g
[0146] Glyceryl monostearate 2.4g
[0147] Tween-801.0g
[0148] 160g of water
[0149] 【Description of preparation process】
[0150] (1) Pass the prescribed amount of Dexibuprofen through an 80-mesh sieve, and mix well with hypromellose (K4M) and hypromellose (K100LV);
[0151] (2) Add an appropriate amount of 85% ethanol to prepare soft materials, and pass through a 24-mesh sieve to obtain wet granules;
[0152] (3) Dry the wet granules in a blast drying oven at 45°C for 4 hours, and pass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com