Preparation method of dexibuprofen amino acid salt and application
A technology of amino acid salt and Dexibuprofen, which is applied in the field of preparation of Dexibuprofen amino acid salt, can solve the problems that patients cannot receive treatment, increase gastrointestinal bleeding, kidney damage, etc. Increase and reduce the stimulating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
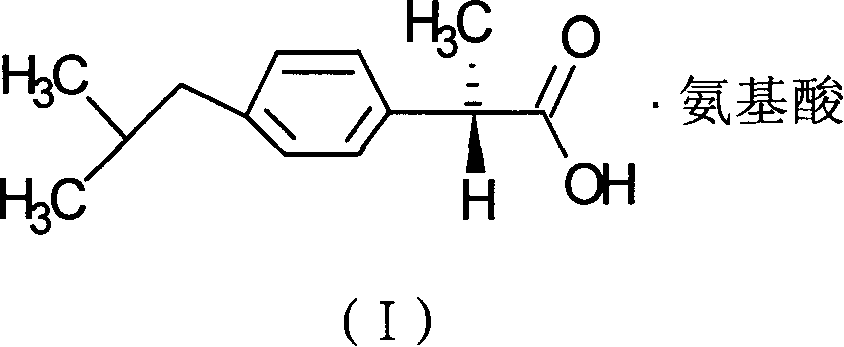
[0022] Compounds of the present invention are:
[0023]
[0024] Chemical name: +2-(4-isobutylphenyl) propionate arginine salt.
[0025] The preparation method is as follows: 100 g of dexibuprofen is placed in a reaction bottle. Add 300ml of ethanol, stir to dissolve, control the temperature between 40-78°C, gradually add 80g of essence, stir to dissolve, keep warm for 30 minutes, after cooling down to room temperature, slowly pour the reaction solution into the prepared acetone solution , a white precipitate precipitated out, stirred for another 10 minutes, put it in the refrigerator for 12 hours, filtered it with suction, washed the crystals with acetone, drained them, put the crystals in an oven at 60°C, and dried them to constant weight to obtain white crystals.
Embodiment 2
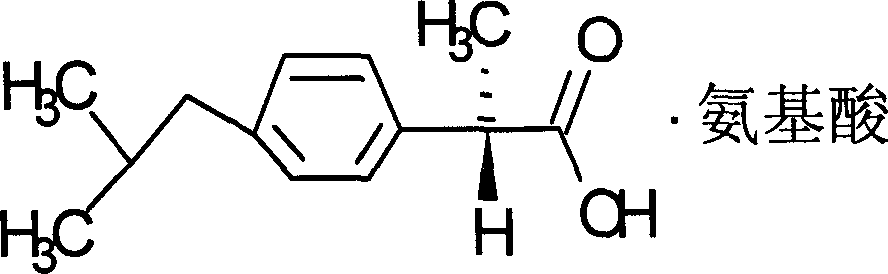
[0027] Compounds of the present invention are:
[0028]
[0029] Chemical name: +2-(4-isobutylphenyl) propionate lysine salt.
[0030] The preparation method is as follows: 100 g of dexibuprofen is placed in a reaction bottle. Add 400ml of ethanol, stir to dissolve, control the temperature between 40-78°C, gradually add 150g of lysyl acid, stir to dissolve, keep it warm for 30 minutes, after cooling down to room temperature, slowly pour the reaction solution into the prepared acetone In the solution, a white precipitate precipitated out, stirred for another 10 minutes, put it in the refrigerator for 12 hours, filtered it with suction, washed the crystal with acetone, drained it, put the crystal in an oven at 60°C, and dried it to constant weight to obtain a white crystal.
Embodiment 3
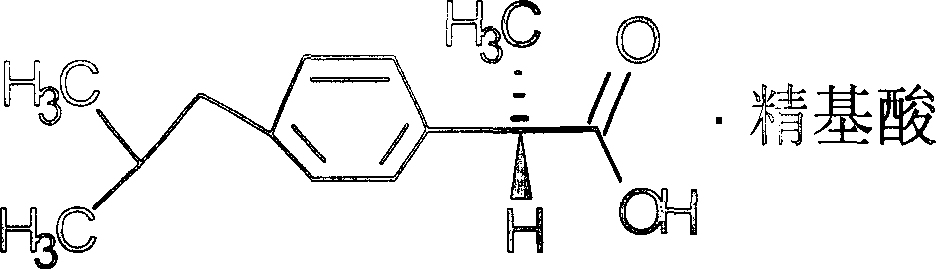
[0032] Compounds of the present invention are:
[0033]
[0034] Chemical name: +2-(4-isobutylphenyl)propionic acid histidine salt.
[0035] Its preparation method is:
[0036] Dexibuprofen 100g is placed in the reaction bottle. Add 300ml of ethanol, stir to dissolve, control the temperature between 40-78°C, gradually add 100g of histidine acid, stir to dissolve, keep it warm for 30 minutes, after cooling down to room temperature, slowly pour the reaction solution into the prepared acetone In the solution, a white precipitate precipitated out, stirred for another 10 minutes, put it in the refrigerator for 12 hours, filtered it with suction, washed the crystal with acetone, drained it, put the crystal in an oven at 60°C, and dried it to constant weight to obtain a white crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com