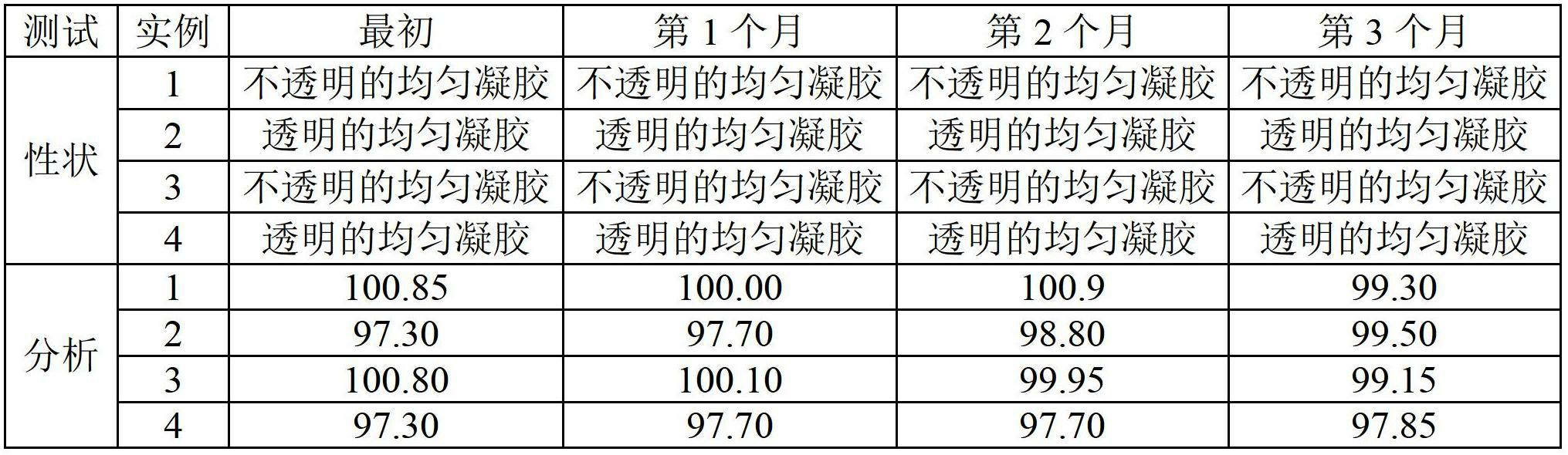
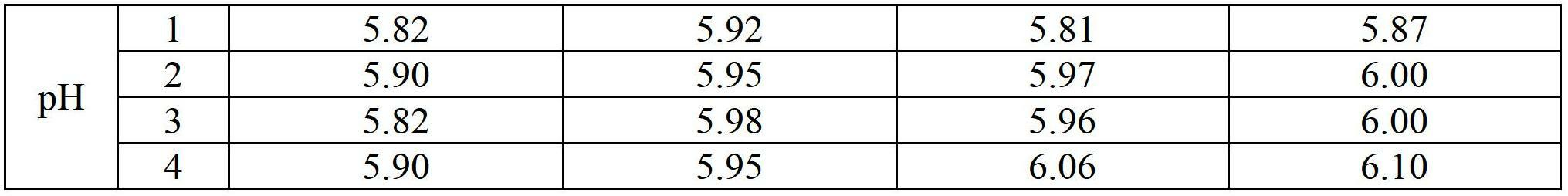
Composition of dexibuprofen transdermal hydrogel
A technology of dexbuprofen and hydrogel, applied in the field of topical pharmaceutical compositions, can solve problems such as poor solubility of ibuprofen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] The invention relates to a locally used pharmaceutical composition containing dexibuprofen, more specifically to a non-alcoholic transdermal hydrogel of dexibuprofen and a preparation method thereof.
[0016] Topical NSAID preparations are commonly used to treat pain and inflammation associated with joints and muscles. The three main advantages of topical NSAIDs over oral treatments for pain and inflammation associated with joints and muscles are:
[0017] i) Higher concentration of NSAID delivered to the desired site;
[0018] ii) Only 1-3% of NSAIDs are absorbed systemically, reducing the likelihood of gastrointestinal upset or ulcers; and
[0019] iii) Low blood levels reduce the incidence of drug interactions.
[0020] A topical formulation of ibuprofen can be used to treat pain and inflammation associated with joints and muscles. Ibuprofen (2-(4-isobutylphenyl)propanoic acid) has one chiral center and therefore exists as two enantiomers, S(+)-ibuprofen (dexibupr...
example 1
[0050] The manufacturing procedure of example 1:
[0051] 1. Disperse Carbopol 971P in pure water with stirring for 15 minutes and allow to soak overnight.
[0052] 2. Dissolve sodium metabisulfite and sodium benzoate in pure water, and then disperse Dexibuprofen in it.
[0053] 3. Dissolve menthol in triethanolamine.
[0054] 4. With constant stirring, add step 3 to step 2 to obtain a clear solution.
[0055] 5. Mix propylene glycol with PEG 400, add this mixture to diethylene glycol monoethyl ether, then add lavender oil and mix well.
[0056] 6. Add step 5 to step 4 and mix well.
[0057] 7. Finally, with constant stirring, add step 6 to step 1 to obtain a homogeneous gel.
[0058] Example-2 (Table-2) Dexibuprofen non-alcoholic transdermal hydrogel prepared by using HPMC as gelling polymer:
[0059] Numbering
example 2
[0060] The manufacturing procedure of example 2:
[0061] 1. Under stirring, disperse HPMC K4M, HPMC E5, Lutrol F68 in pure water for 15 minutes, and let it soak overnight.
[0062] 2. Add simethicone to step 1 and mix well.
[0063] 3. Dissolve sodium metabisulfite and sodium benzoate in water, and then disperse Dexibuprofen in it.
[0064] 4. Dissolve menthol in triethanolamine.
[0065] 5. With constant stirring, add step 4 to step 3 to get a clear solution.
[0066] 6. Mix Propylene Glycol, PEG 400 and add this mixture to Diethylene Glycol Monoethyl Ether followed by Lavender Oil and mix well.
[0067] 7. Add step 6 to step 5.
[0068] 8. With constant stirring, add step 7 to step 2 to obtain a homogeneous gel.
[0069] Example-3 (Table-3) Dexibuprofen non-alcoholic transdermal hydrogel prepared by using Carbopol as gelling polymer:
[0070] Numbering
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com