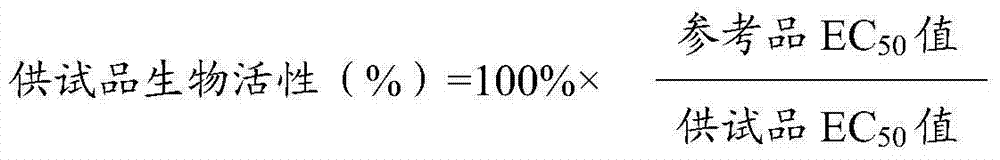Cytokine TNF-alpha bioactivity evaluation in-vitro test method
A cytokine and active body technology, applied in the field of biomedicine, can solve the problem of difficulty in meeting the needs of TNF-α clone screening, production release and stability research, difficulty in ensuring the stability and reliability of test results, and lack of detection system methods. To solve problems such as scientific verification research, etc., to achieve the effect of convenient high-throughput operation, easy promotion and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 In vitro detection method for evaluating the biological activity of cytokine TNF-α
[0044] Using the reference product and the TNF-α test product with a titer of 75% and 125% as materials, the biological activity of the TNF-α test product at a titer of 75% and 125% was investigated using the steps of the above detection method.
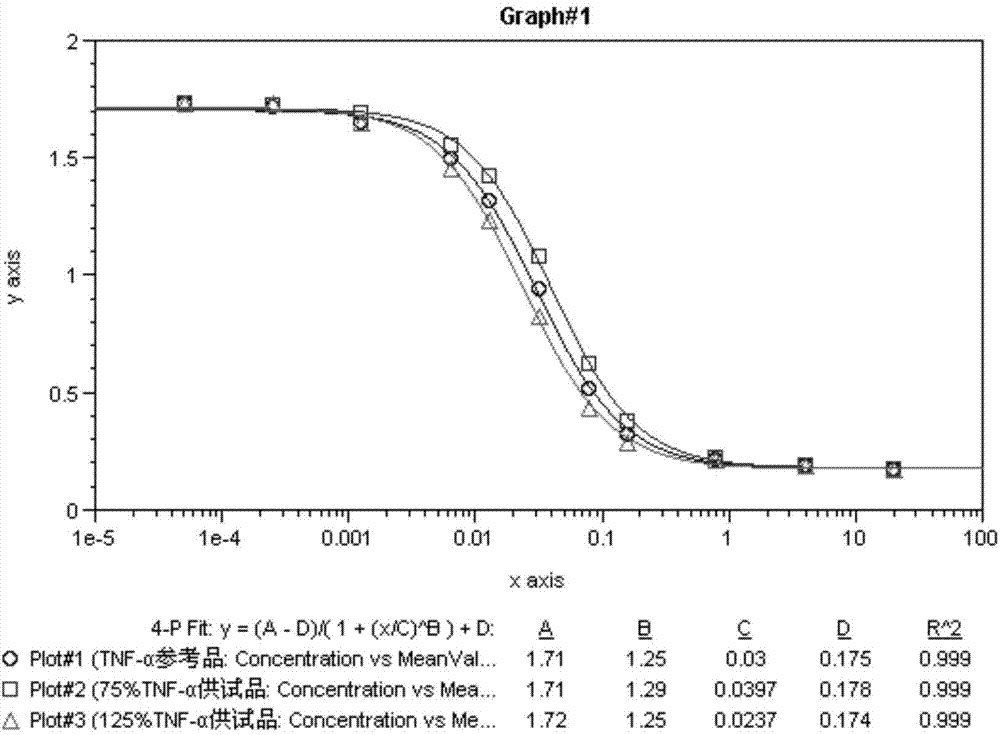
[0045] The result is figure 1 As shown, the 4 parameters of the experimental curve of the TNF-α reference product and the test product are well fitted, and the curve fitting constant R 2 All met> 0.98, and the CV% of the OD value detected by 2 multiple holes at different concentrations of the added drug met 50 Comparison of the values, the cytotoxic biological activity of the test product at the 75% titer level was 76%, and the cytotoxic biological activity of the test product at the 125% titer level was 126%, both close to the theoretical value.
Embodiment 2
[0046] Example 2 Accuracy Evaluation Test
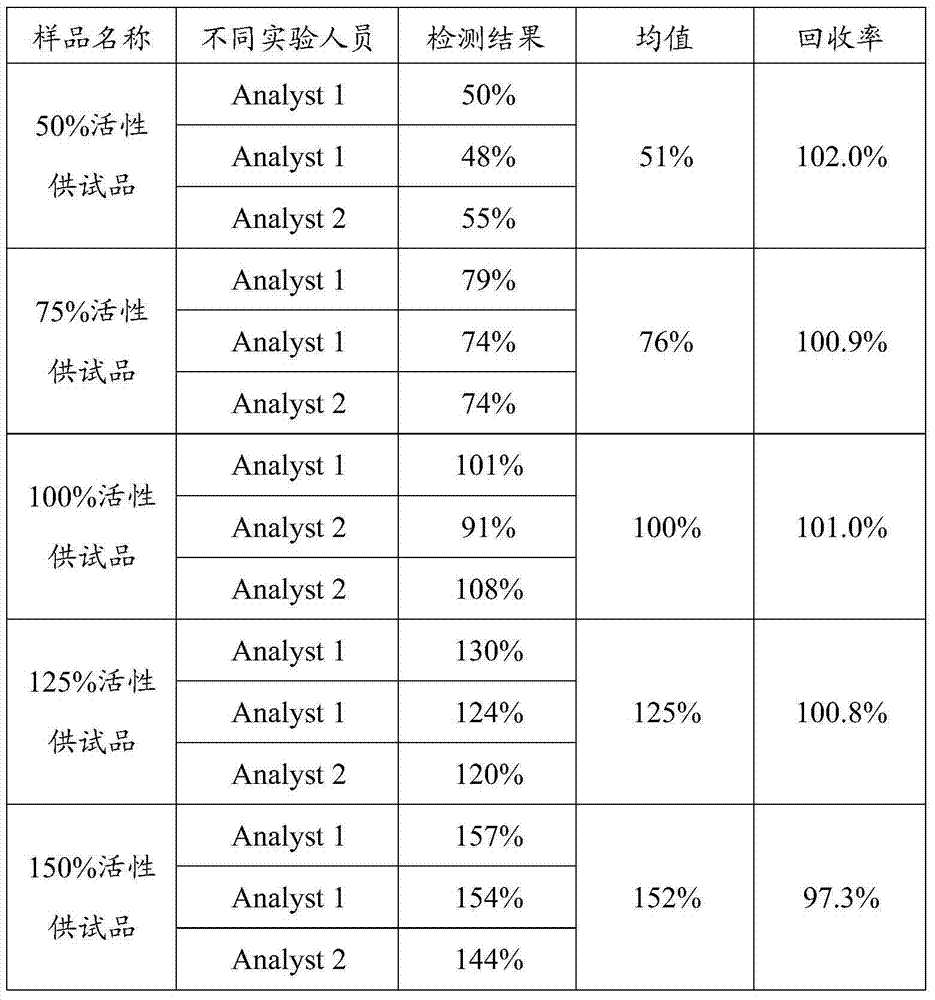
[0047] Using the reference product (same as Example 1) and the test product with different potency levels (50%, 75%, 100%, 125%, 150%) as materials, 2 experimenters (Analyst 1, 2) independently adopted The above-mentioned detection method detects the cytotoxic biological activity of the test products at these 5 potency levels.
[0048] The results are shown in Table 1. For 5 test products with potency levels of 50%, 75%, 100%, 125%, and 150% respectively, the test results of these test products by 2 experimenters using the method of the present invention are all satisfactory 4 The fitting constant R of the parameter fitting curve 2 >0.98, the CV% of 2 multiple holes meets <15%, and the average deviation (recovery rate) of the actual measured value of the test product with different potency levels by different personnel meets the theoretical value in the range of 95%-105% Inside. It shows that when detecting the cytotoxic biological acti...
Embodiment 3
[0051] Example 3 Precision Evaluation Test
[0052] Using reference products (same as Example 1) and test products with different potency levels as materials, the precision of the method of the present invention was evaluated. Two experimenters (Analyst 1, 2) used the above-mentioned detection method steps to test the cytotoxic biological activity of 5 test products with potency levels of 50%, 75%, 100%, 125%, and 150%, each in parallel Experiment 3 times to investigate the repeatability of the method. Two experimenters (Analyst 1, 2) used the above detection method steps to test the cytotoxic biological activity of the test product at the 75% titer level within 3 different days. The middle of the investigation method Precision.
[0053] As shown in Table 2, using the above detection method, different experimenters performed three parallel tests on each of the five potency levels of the test product, and the results all met the fitting constant R of the 4-parameter fitting curve o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com