Preparation method of dexibuprofen sustained-release agent
A technology of slow-release agent and montmorillonite, which is applied in the field of preparation of Dexibuprofen sustained-release agent, can solve the problems of many times of administration, many types of synthetic products, adverse reactions, etc., and achieve accurate and detailed data and process flow. Short, non-polluting effect on the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0063] The present invention will be further described below in conjunction with accompanying drawing:
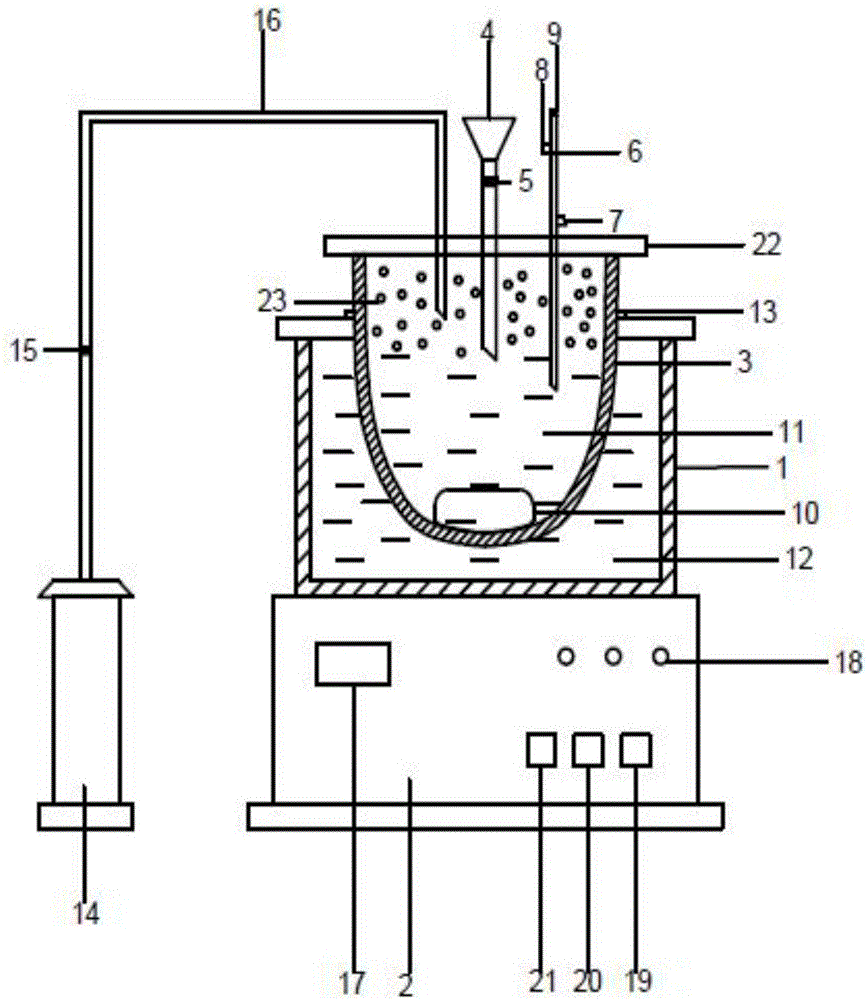
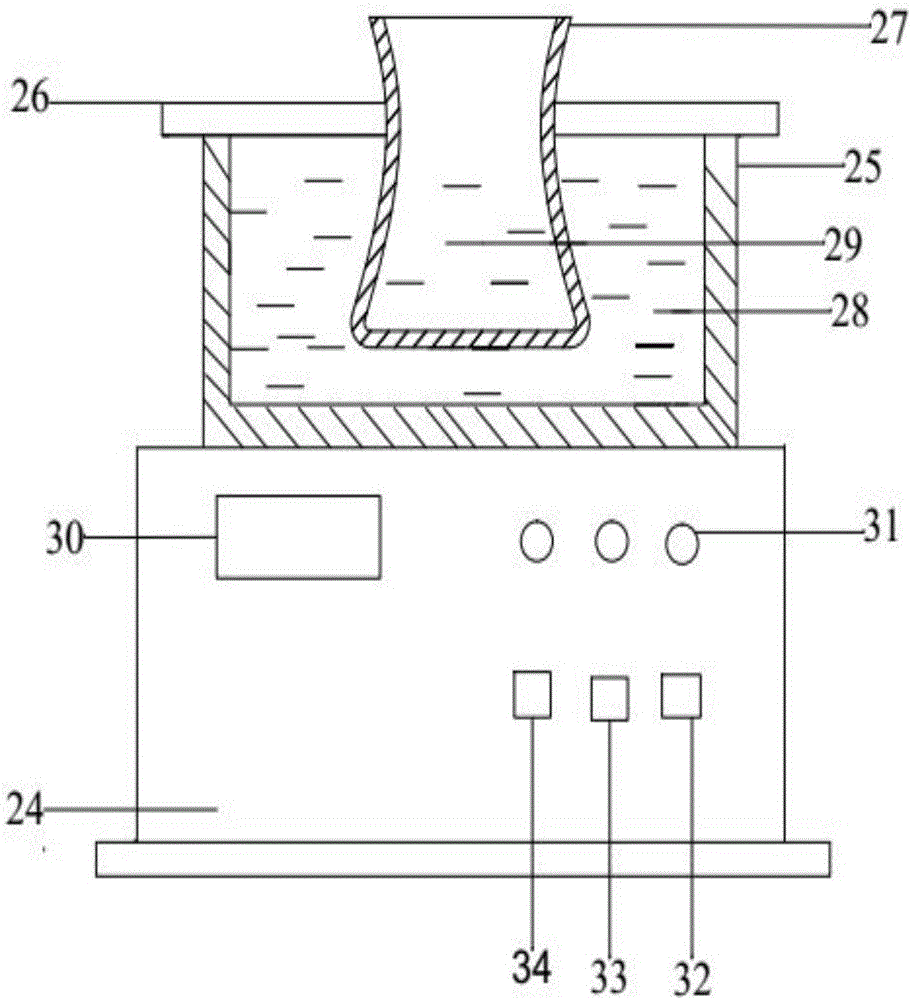
[0064] figure 1 As shown, in order to prepare the state diagram of the montmorillonite mixture, the positions of each part must be correct, the ratio should be proportioned, and the operations should be performed in sequence.
[0065]Quantities of chemicals used in preparation are determined in pre-set ranges in grams, milliliters, centimeters 3 is the unit of measurement.
[0066] The preparation of the montmorillonite mixture is carried out in a three-necked flask, which is completed under the conditions of heating in a water bath, argon protection, magnetic stirring, and water circulation and condensation;
[0067] The water bathtub 1 is rectangular, and there is a three-necked flask 3 on the top of the water bathtub 1, and is fixed by a fixed seat 13; the bottom of the water bathtub 1 is an electric control box 2; Argon tube 16, liquid addition funnel 4, control valv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com