Catalyst for alkane dehydrogenation and device
A technology of alkane dehydrogenation and catalyst, which is applied in the field of low-carbon alkane dehydrogenation catalyst and alkane catalytic dehydrogenation device, which can solve the problems of high investment and catalyst use cost, frequent catalyst regeneration, and expensive Pt, etc., and achieve long regeneration cycle , high selectivity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
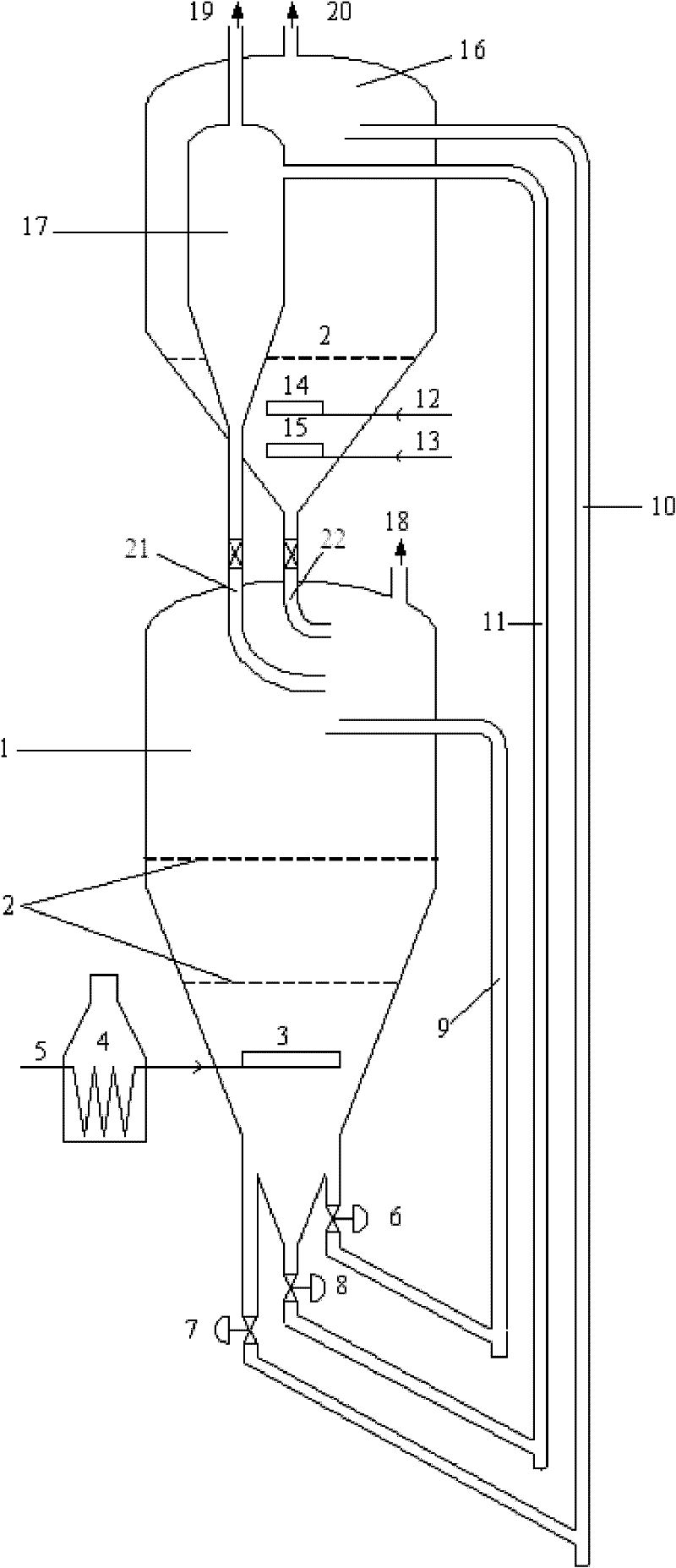
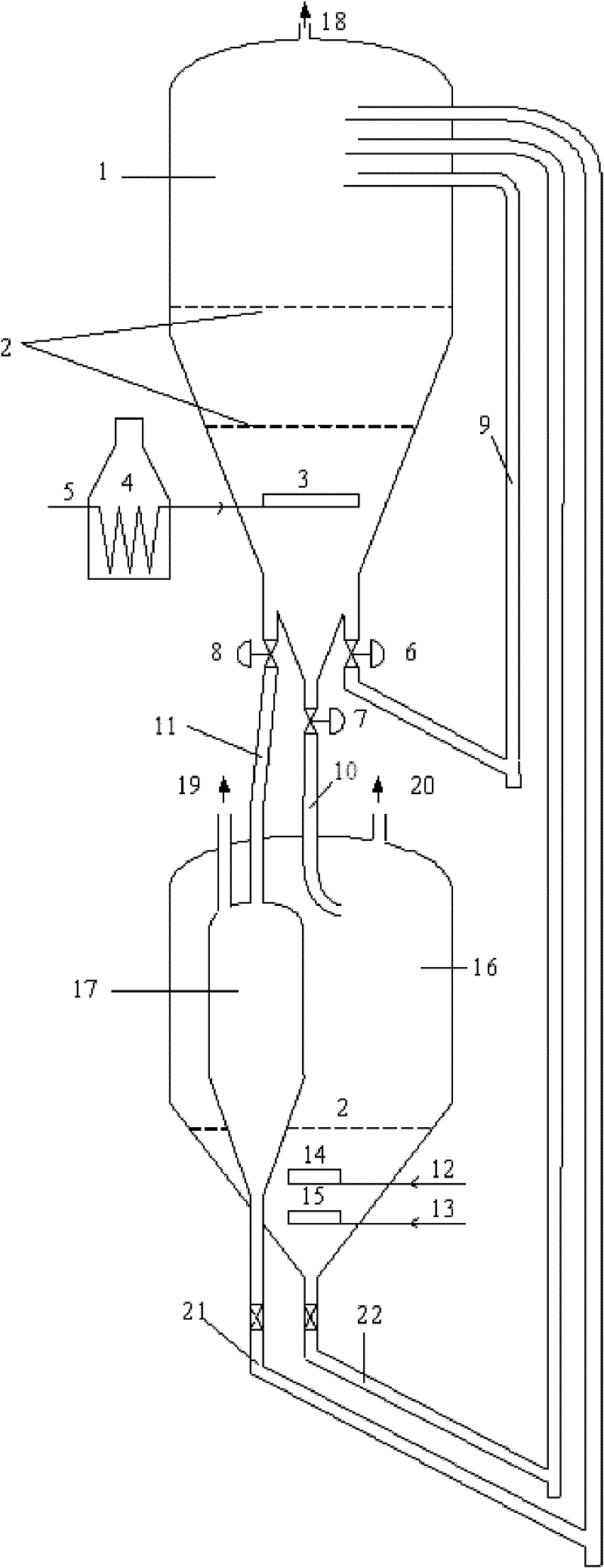
Image
Examples
Embodiment 1
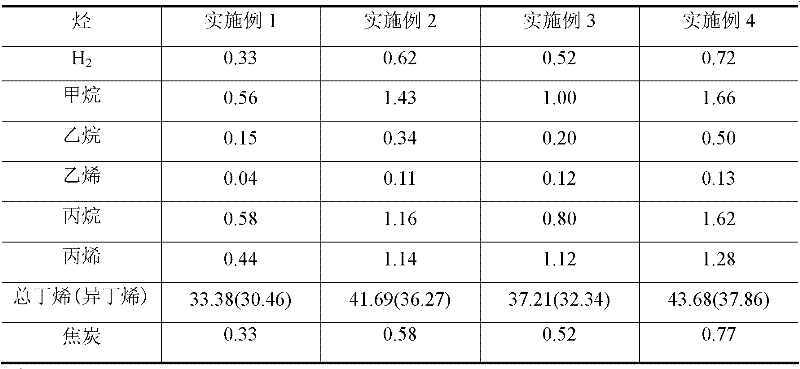
[0082] Add 352.9 g of deionized water to 88.23 g of pseudo-boehmite, stir well in a water bath at 80°C, and add hydrochloric acid to adjust the pH value to about 3. 20g tin oxide (SnO 2 ) and 15g titanium oxide (TiO 2 ) powder into the prepared gel, then add 50g of deionized water, mechanically stir evenly, dry at 120°C for 8h, then roast at 600°C for 10h, cool, crush and sieve. Weigh 8.52g of Ba(NO 3 ) 2 , dissolved in 20g deionized water. Ba(NO) was impregnated on the prepared catalyst 3 ) 2 solution, dried at 140°C for 9h, and then calcined at 600°C for 12h. The evaluation results of the catalyst showed that the conversion rate of isobutane was 35.81wt%, the yield of isobutene was 30.46wt%, and the selectivity of isobutene was 85.06wt%.
Embodiment 2
[0084] Add 352.9 g of deionized water to 88.23 g of pseudo-boehmite, stir well in a water bath at 80° C., add nitric acid to adjust the pH value to about 3. 20g tin oxide (SnO 2 ), 10g titanium oxide (TiO 2 ) and 5g gallium oxide (Ga 2 o 3 ) powder into the prepared gel, then add 50g of deionized water, mechanically stir evenly, dry at 120°C for 7h, then roast at 600°C for 10h, cool and crush and sieve. Weigh a certain mass of Ca(NO 3 ) 2 , calculated according to the mass of its oxide as 5g, dissolved in 20g of deionized water and stirred evenly. The prepared catalyst was impregnated with Ca(NO 3 ) 2 solution, dried at 140°C for 8h, and then calcined at 600°C for 12h. The catalyst evaluation results showed that the conversion rate of isobutane was 47.07wt%, the yield of isobutene was 36.27wt%, and the selectivity of isobutene was 77.06wt%.
Embodiment 3
[0086] Add 352.9 g of deionized water to 88.23 g of pseudoboehmite, stir well in a water bath at 80° C., and add nitric acid to adjust the pH to about 3.5. 20g tin oxide (SnO 2 ), 10g titanium oxide (TiO 2 ) and 5g of magnesium oxide (MgO) powder were added to the prepared gel, then 50g of deionized water was added, mechanically stirred evenly, dried at 120°C for 6h, then calcined at 600°C for 10h, cooled, crushed and sieved. Weigh a certain mass of LiNO 3 , calculated according to the mass of its oxide as 5g, dissolved in 20g of deionized water and stirred evenly. Impregnation of LiNO on the catalyst 3 solution, dried at 140°C for 10h, and then calcined at 600°C for 12h. The catalyst evaluation results showed that the conversion rate of isobutane was 41.49wt%, the yield of isobutene was 32.34wt%, and the selectivity of isobutene was 77.95wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conversion rate per pass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com