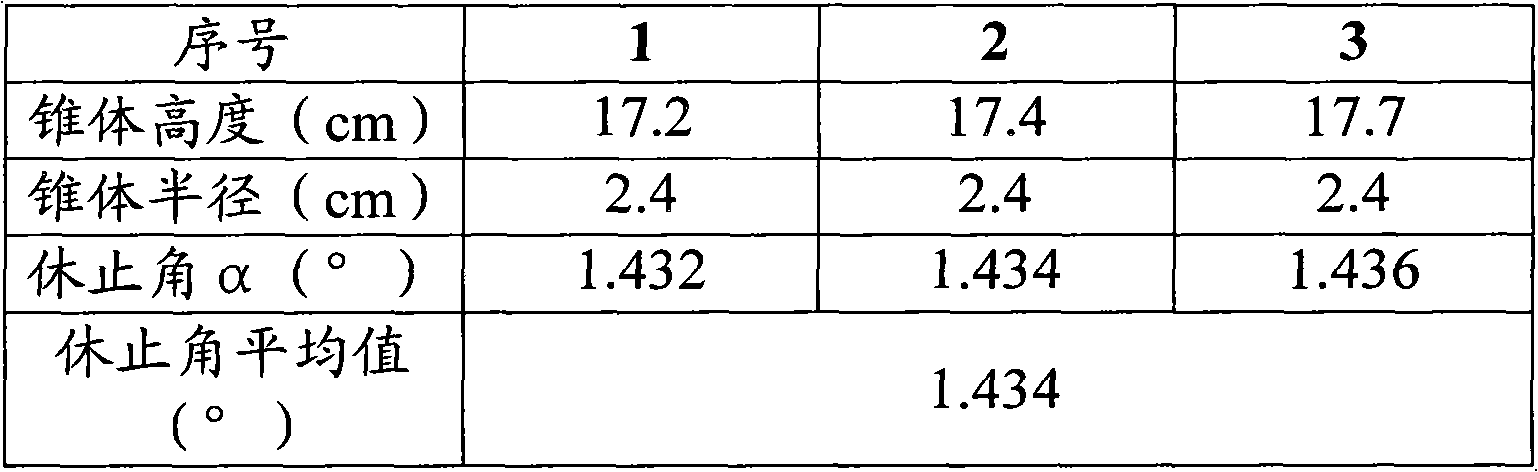Dexibuprofen particle and preparation method thereof
A granule and sodium carbonate technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of long time required for low-temperature drying, unqualified microbial limits, and difficult moisture control and other problems, to achieve the effect of stable and controllable quality, suitable for industrial production, and easy control of microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Pass the dextrobuprofen, sodium carbonate, and aspartam through 80-mesh sieves respectively, and sucrose after crushing and pass through 80-mesh sieves for later use.
[0043] Weigh the raw materials of the following prescription quantities:
[0044] Dexibuprofen 200g
[0046] Sucrose 2480g
[0047] Aspartame 60g
[0048] 75% ethanol 860g
[0049] Lychee essence 10g.
[0050] Fully mix aspartam, dextrobuprofen, and sodium carbonate to obtain a mixture. Divide sucrose into 3 parts of equal weight, add the above mixture in 3 times, mix for 10 minutes after each addition, and mix well. Finally, add 75% ethanol to make soft material, use 20 mesh sieve to granulate, blow dry at 60°C for 2 hours, spray lychee essence, mix, and obtain dextroibuprofen granules after 18 mesh sieve granulation, intermediate After passing the inspection, use aluminum-plastic composite bags to pack into 14600 bags according to 200mg / bag, and pack to get the fini...
Embodiment 2
[0052] Pass the dextrobuprofen, sodium carbonate, and aspartam through 80-mesh sieves respectively, and sucrose after crushing and pass through 80-mesh sieves for later use.
[0053] Weigh the raw materials of the following prescription quantities:
[0054] Dexibuprofen 100g
[0055] Sodium carbonate 300g
[0056] Sucrose 2360g
[0057] Aspartame 14g
[0058] 75% ethanol 460g
[0059] Orange essence 5g.
[0060] Fully mix aspartam, dextrobuprofen, and sodium carbonate to obtain a mixture. Divide sucrose into 3 parts of equal weight, add the above mixture in 3 times, mix for 10 minutes after each addition, and mix well. Finally, add 75% ethanol to make a soft material, use 20 mesh sieve to granulate, then blow dry at 70°C for 1.5h, spray into orange essence, and mix them all. After passing the intermediate inspection, use aluminum-plastic composite bags to divide into 13480 bags according to 200 mg / bag, and pack to obtain the finished product.
Embodiment 3
[0062] Pass the dextrobuprofen, sodium carbonate, and aspartam through 80-mesh sieves respectively, and sucrose after crushing and pass through 80-mesh sieves for later use.
[0063] Weigh the raw materials of the following prescription quantities:
[0064] Dexibuprofen 400g
[0065] Sodium carbonate 500g
[0066] Sucrose 4360g
[0067] Aspartame 60g
[0068] 75% ethanol 1300g
[0069] Green Apple Flavor 20g.
[0070] Fully mix aspartam, dextrobuprofen, and sodium carbonate to obtain a mixture. Divide sucrose into 3 parts of equal weight, add the above mixture in 3 times, mix for 10 minutes after each addition, and mix well. Finally, add 75% ethanol to make a soft material, use a 20-mesh sieve to granulate, and then dry it by air at 80°C for 1 hour. After passing the physical examination, use aluminum-plastic composite bags to pack into 25280 bags according to 200 mg / bag, and pack to get the finished product.
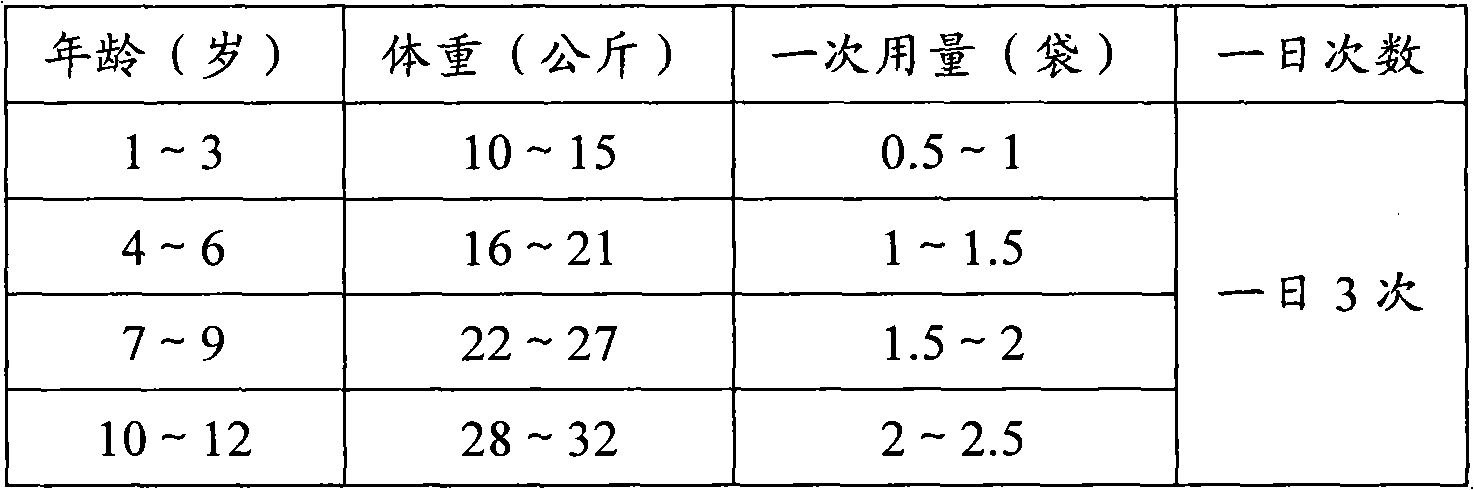
[0071] The Dexibuprofen granule that embodiment 1~3 is made...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com