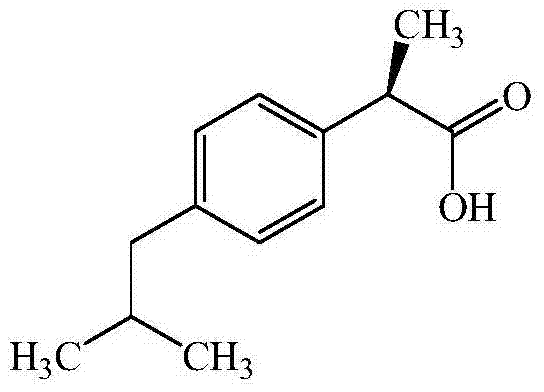
Dexibuprofen purifying method
A refining method and organic solvent technology, applied in the field of refining ibuprofen, can solve the problems of high cost of sodium alcoholate, high impurity content, low yield, etc., and achieve simple and easy-to-control process steps, high product purity, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add 50 g of the crude product of Dexibuprofen into the reaction flask, add 500 mL of acetone and stir, heat at 30 ° C until the system is clear, add dropwise 10% sodium hydroxide solution to adjust the pH to 9.0, after the drop is complete, keep stirring for 20 minutes, Cool down to -10°C, stir and crystallize for 4 hours, and filter to obtain Dexibuprofen sodium salt. Dissolve the sodium salt in 400mL of pure water, add 200mL of n-hexane, add hydrochloric acid dropwise under stirring to adjust the pH to 1.1, extract, separate the liquids, extract the water phase with 200mL of n-hexane again, combine the organic phases, and wash two times with 200mL of pure water. Once, the organic phase was distilled under reduced pressure to obtain the pure product of Dexibuprofen, with a yield of 76%. After HPLC detection, the maximum single impurity is 0.07%, the total impurity is 0.5%, and the ee value is 99.5%.
Embodiment 2
[0046] Add 50 g of the crude product of Dexibuprofen into the reaction flask, add 250 mL of ethanol and stir, heat at 45 ° C until the system is clear, add dropwise 20% sodium hydroxide solution to adjust the pH to 9.5, after the drop is complete, keep stirring for 20 minutes, Cool down to 0°C, stir and crystallize for 2 hours, and filter to obtain Dexibuprofen sodium salt. Dissolve the sodium salt in 400mL of pure water, add 200mL of n-hexane, add concentrated hydrochloric acid dropwise under stirring to adjust the pH=1.5, extract, separate the liquids, extract the water phase with 200mL of n-hexane again, combine the organic phases, and wash with 200mL of pure water Twice, the organic phase was distilled under reduced pressure to obtain the pure product of Dexibuprofen with a yield of 81%. After HPLC detection, the maximum single impurity is 0.08%, the total impurity is 0.4%, and the ee value is 99.6%. · Ibuprofen ethyl ester impurity was detected.
Embodiment 3
[0048]Add 50g of the crude product of Dexibuprofen into the reaction flask, add 200mL of acetone and stir, heat at 50°C until the system is clear, add dropwise 25% sodium hydroxide solution to adjust the pH to 9.8, after dropping, keep stirring for 20 minutes, Cool down to 0°C, stir and crystallize for 2 hours, and filter to obtain Dexibuprofen sodium salt. Dissolve the sodium salt in 400mL of pure water, add 200mL of n-hexane, add concentrated hydrochloric acid dropwise under stirring to adjust the pH=3.1, extract, separate the liquids, extract the water phase with 200mL of n-hexane again, combine the organic phases, and wash with 200mL of pure water Twice, the organic phase was distilled under reduced pressure to obtain the pure product of Dexibuprofen, with a yield of 82%. After HPLC detection, the maximum single impurity is 0.05%, the total impurity is 0.21%, and the ee value is 99.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com