Preparation method of dexibuprofen
A technology of dexbuprofen and meglumine, which is applied in the field of preparation of dexbuprofen, can solve the problems of low melting point of dextro-amine salt, content of dextro-ibuprofen levorotate exceeding the qualified standard and the like, and achieves high molar yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
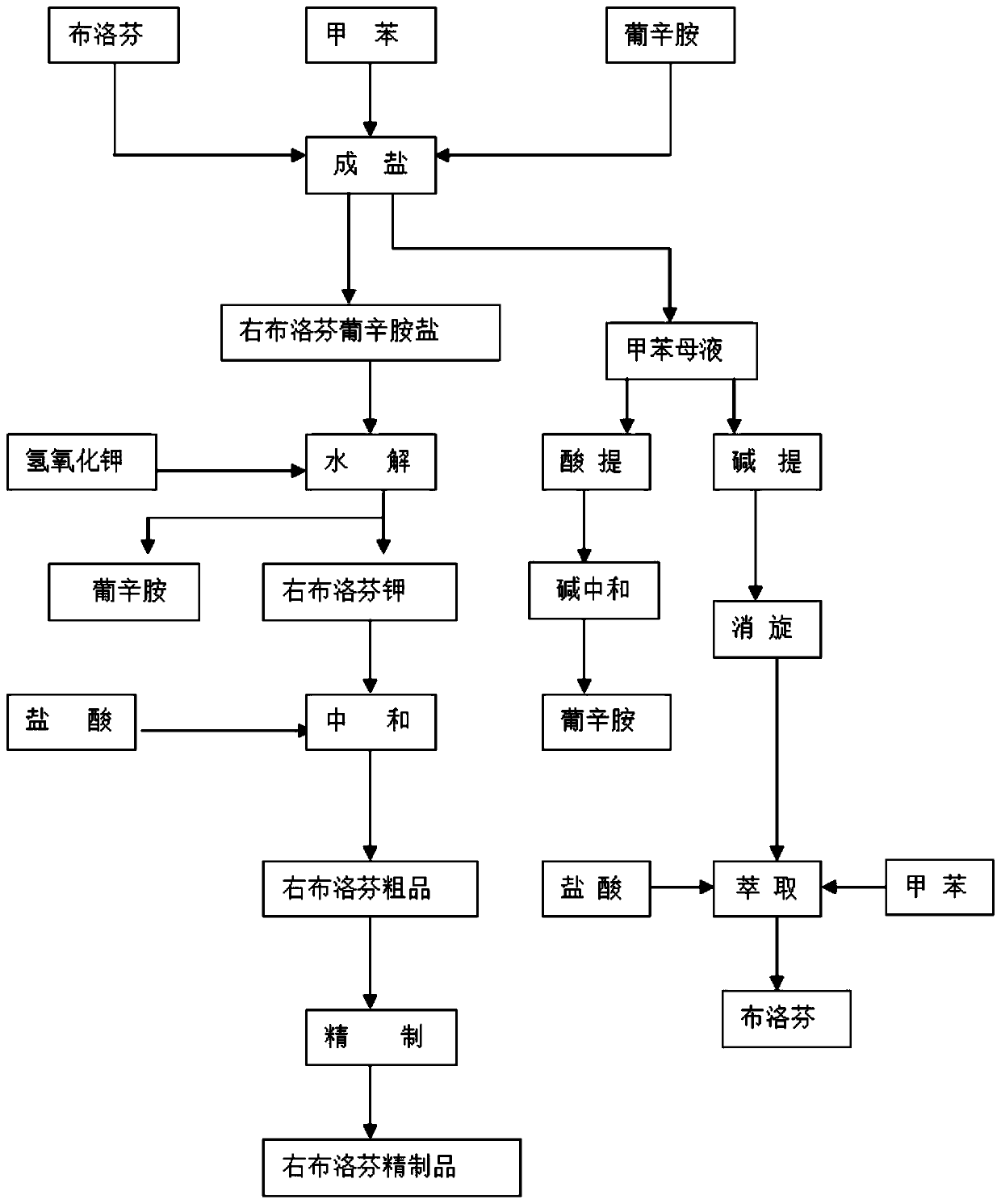
[0017] A preparation method of Dexibuprofen, said method comprising:
[0018] 1. Preparation of amine salt:
[0019] 1. Put the amount of toluene (2448L) into the reaction kettle according to the feeding ratio, add about 50L hydrochloric acid solution (14-18%), stir for more than 10 minutes, let stand for more than 10 minutes, and divide the hydrochloric acid layer; then add about 200L For drinking water, stir for more than 10 minutes, let it stand for more than 10 minutes, and separate the water layer.
[0020] 2. Put in 315kg of ibuprofen and 216kg of octylglucamine according to the ratio, seal the hole cover, stir and heat up to 76-80°C, and keep it warm for more than 0.5 hours.
[0021] 3. Transfer the material to an amine salt crystallization kettle, add about 4.5L of purified water, and then cool down to crystallize. After the temperature drops to 18-22°C, put the material in a centrifuge to dry (10-15min), and then wash it with toluene 1 time (30~50L), rinse once (30~...
Embodiment 2
[0034] A preparation method of Dexibuprofen, said method comprising:
[0035] 1. Preparation of amine salt:
[0036] 1. Put the process amount of toluene (2362.5L) into the reaction kettle according to the feeding ratio, add about 50L hydrochloric acid solution (14-18%), stir for more than 10 minutes, let stand for more than 10 minutes, and separate the hydrochloric acid layer; then add about 200L drinking water, stir for more than 10 minutes, let stand for more than 10 minutes, and separate the water layer.
[0037] 2. Put in 315kg of ibuprofen and 204.75kg of octylglucamine according to the ratio, seal the hole cover, stir and heat up to 76-80°C, and keep it warm for more than 0.5 hours.
[0038] 3. Transfer the material to an amine salt crystallization kettle, add about 3.15L of purified water, and then cool down to crystallize. After the temperature drops to 18-22°C, put the material in a centrifuge to dry (10-15min), and then wash it with toluene 1 time (30~50L), rinse ...
Embodiment 3
[0051] A preparation method of Dexibuprofen, said method comprising:
[0052] 1. Preparation of amine salt:
[0053] 1. Put the process amount of toluene (2520L) into the reaction kettle according to the feeding ratio, add about 50L hydrochloric acid solution (14-18%), stir for more than 10 minutes, let stand for more than 10 minutes, separate the hydrochloric acid layer; then add about 200L For drinking water, stir for more than 10 minutes, let it stand for more than 10 minutes, and separate the water layer.
[0054] 2. Put in 315kg of ibuprofen and 236.25kg of octylglucamine according to the ratio, seal the hole cover, stir and heat up to 76-80°C, and keep it warm for more than 0.5 hours.
[0055] 3. Transfer the material to the amine salt crystallization kettle, add about 6.3L of purified water, and then cool down to crystallize. After the temperature drops to 18-22°C, put the material in a centrifuge to dry (10-15min), and then wash it with toluene 1 time (30~50L), rinse...
PUM
| Property | Measurement | Unit |
|---|---|---|
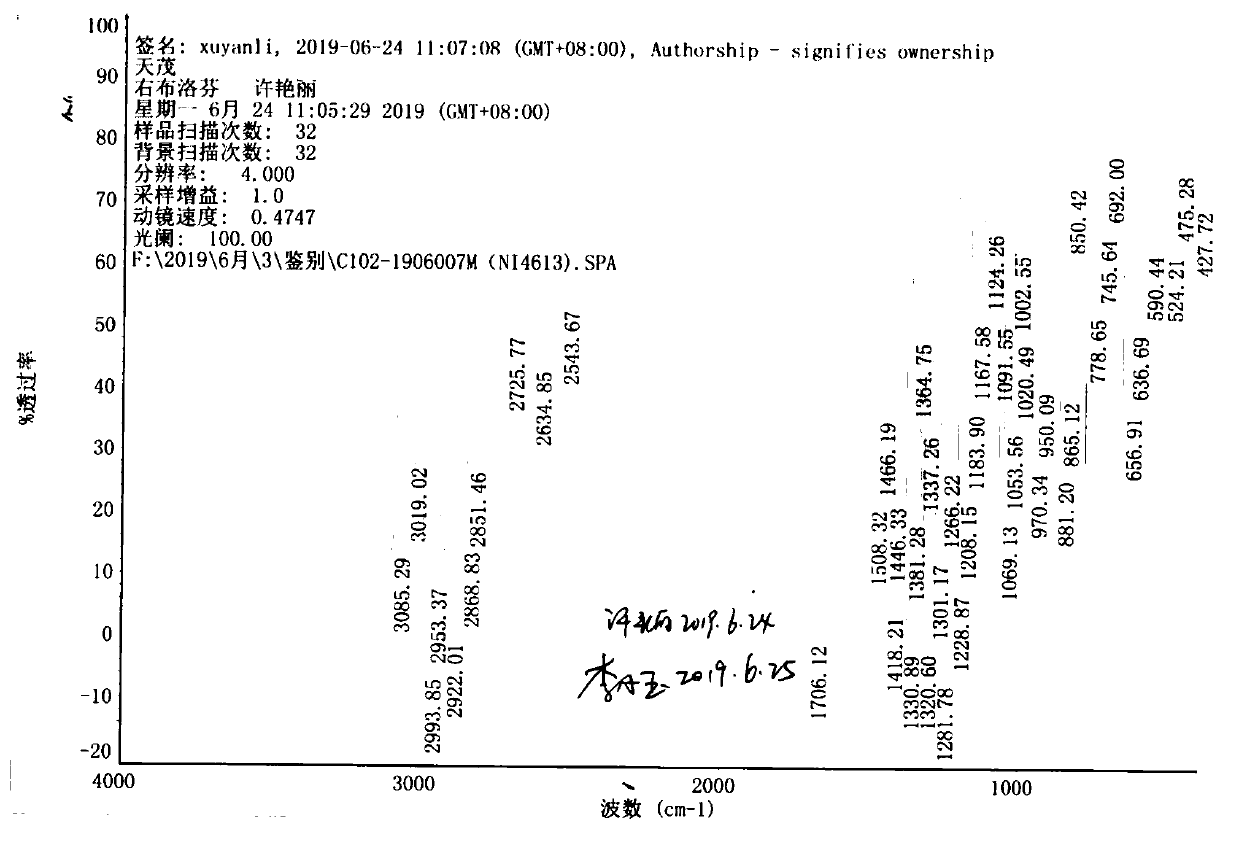
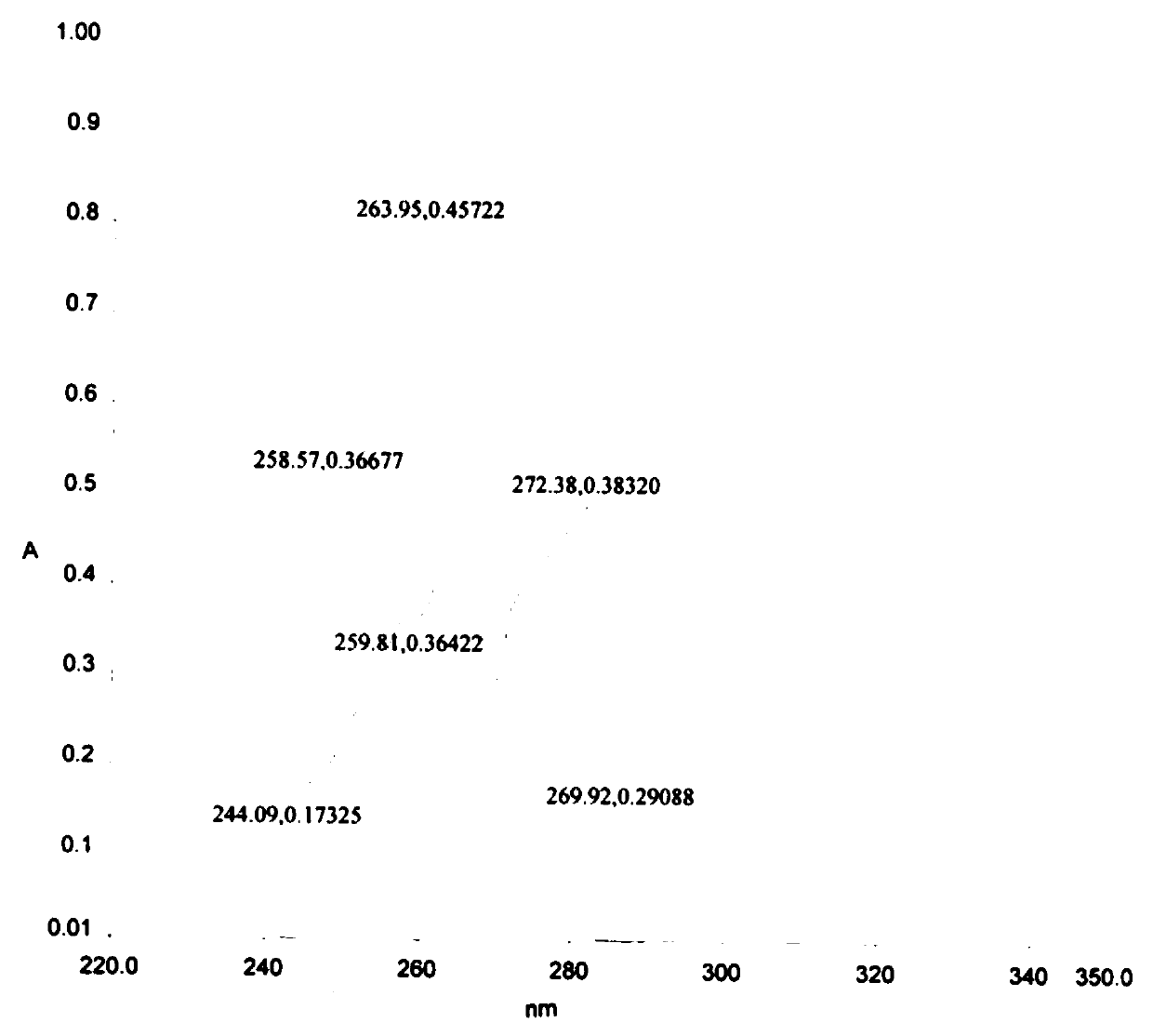
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com