Method for determining dexibuprofen related matter
A technology for related substances and impurities, which is applied in the field of determination of dextroibuprofen related substances, can solve the problems of no literature report and few related substances analysis methods, and achieves the effects of good durability and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
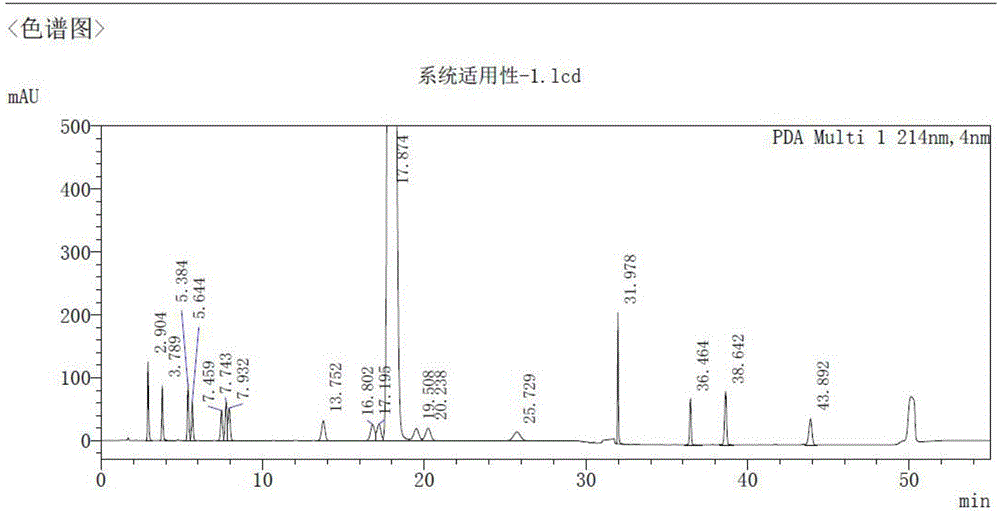
[0014] The reversed-phase high-performance liquid chromatography of embodiment 1 Dexibuprofen related substances is determined
[0015] Chromatographic conditions and system adaptability: Octadecylsilane bonded silica gel is used as filler (SHISEIDO CAPCELLC18 MGⅡ 3μm 4.6×150mm), detection wavelength is 214nm, column temperature is 25°C, and flow rate is 1.0mL / min. Mobile phase A is: 0.5ml phosphoric acid plus 340ml acetonitrile plus 600ml water to a 1000ml measuring bottle, mix well, add water to dilute to the mark, phase B: acetonitrile, the gradient program is shown in the table below. Gradient elution was carried out according to the following mobile phase conditions:
[0016] time (min) mobile phase A mobile phase B 0 80 20 25 80 20 25.1 5 95 45 5 95 45.1 80 20 55 80 20
[0017] The following items related to the detection method of the substance have been verified:
[0018] 1. System suitability experiment
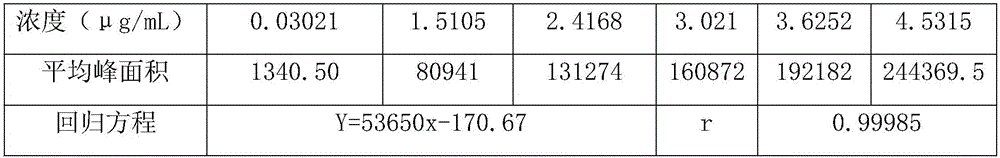
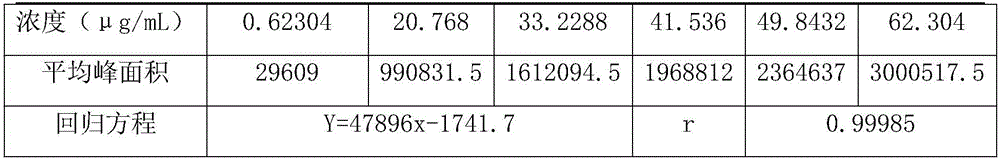
[0019] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com