Slow-release dry dexibuprofen suspension and preparation method thereof
A technology of dry suspension and dexibuprofen, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc., can solve the problem that the slow-release effect of the suspension needs to be further improved, and avoid blood drug concentration Peak-valley phenomenon, improving medication compliance, good taste-masking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
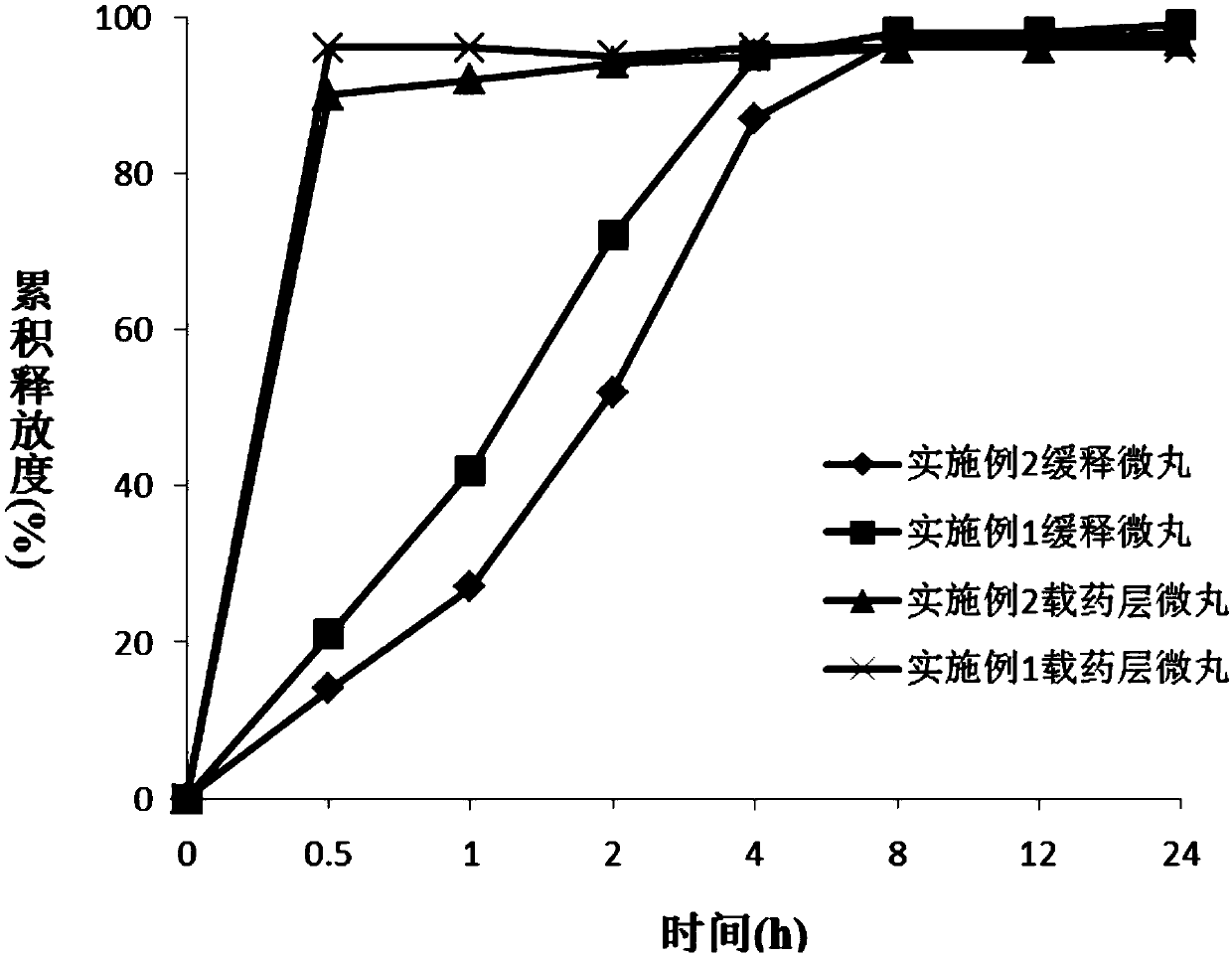
[0047] Embodiment 1: Preparation of Dexibuprofen sustained-release pellets
[0048] Prepare Dexibuprofen sustained-release pellets according to the formula in table 1:
[0049] Table 1 Dexibuprofen sustained-release pellet formula
[0050]
[0051] The specific preparation process of the above-mentioned Dexibuprofen sustained-release pellets is as follows:
[0052] 1. Drug-loaded layer coating:
[0053] A. Add Poloxamer 188 to 95% ethanol solution, stir slowly to make it fully dissolved, then add Dexibuprofen, stir evenly to obtain a clear drug-loaded layer coating solution, set aside.
[0054] B, open the fluidized bed coating machine, put microcrystalline cellulose into the fluidized bed coating machine, use the above drug-loaded layer coating liquid to carry out the drug-loaded layer coating operation to it (coating parameters: material temperature is 28~31℃, inlet air temperature is 38~42℃, atomization pressure is 0.05~0.07MPa, liquid spraying speed is 1.0~1.5mL / min)...
Embodiment 2
[0058] Embodiment 2: Preparation of Dexibuprofen sustained-release pellets
[0059] Prepare Dexibuprofen sustained-release pellets according to the formula in table 2:
[0060] Table 2 Dexibuprofen sustained-release pellet formula
[0061]
[0062] The specific preparation process of the above-mentioned Dexibuprofen sustained-release pellets is as follows:
[0063] 1. Drug-loaded layer coating:
[0064] A. Add PVP-K30 to 95% ethanol solution, stir slowly to make it fully dissolved, then add Dexibuprofen, stir evenly to obtain a clear drug-loaded layer coating solution, set aside.
[0065] B, open the fluidized bed coating machine, put microcrystalline cellulose into the fluidized bed coating machine, use the above drug-loaded layer coating liquid to carry out the drug-loaded layer coating operation to it (coating parameters: material temperature is 28~31℃, inlet air temperature is 38~42℃, atomization pressure is 0.05~0.07MPa, liquid spraying speed is 1.0~1.5mL / min), afte...
Embodiment 3
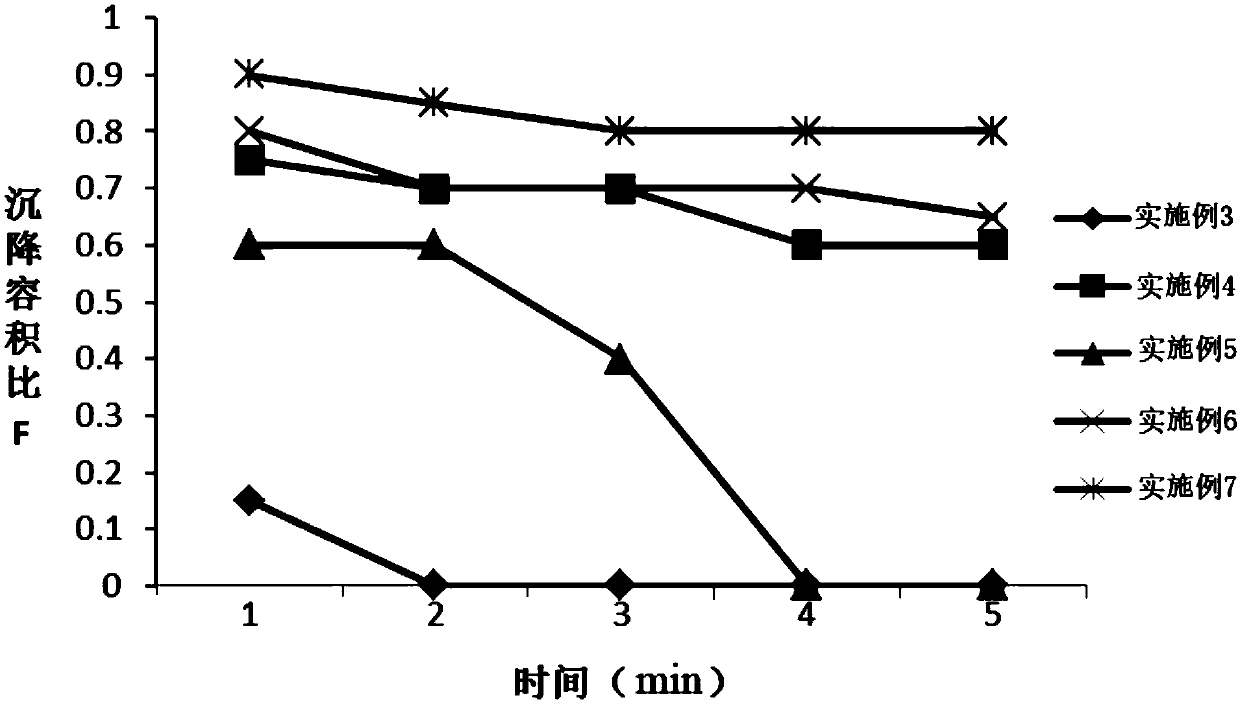
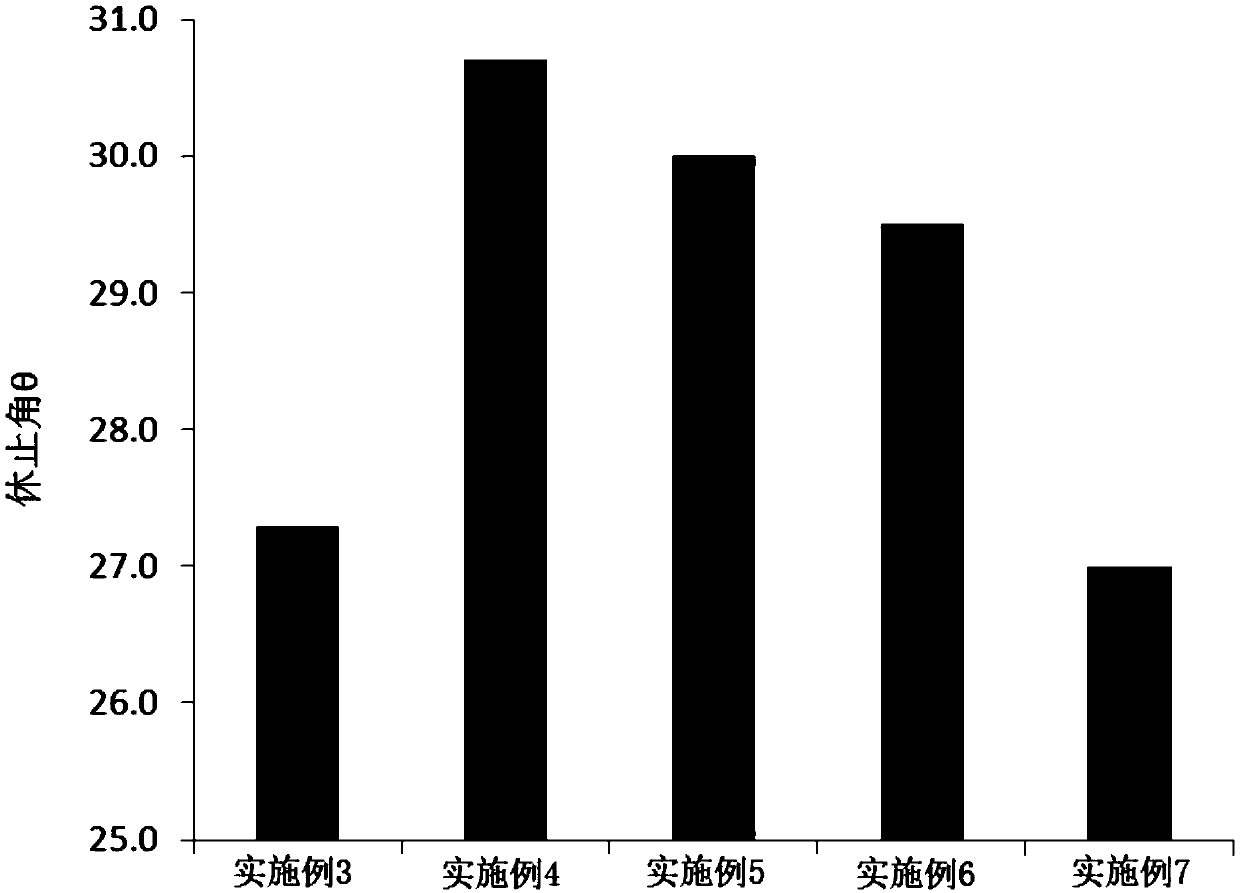
[0071] Embodiment 3: Preparation of Dexibuprofen Sustained Release Dry Suspension
[0072] Prepare Dexibuprofen Sustained Release Dry Suspension according to the formula in Table 3:
[0073] Table 3 Dexibuprofen Sustained Release Dry Suspension Formulation
[0074]
[0075] The concrete preparation process of above-mentioned Dexibuprofen slow-release dry suspension is as follows:
[0076] 1. Preparation of diluted granules:
[0077] Poloxamer 188 was dissolved in water to obtain solution A. Mix sucrose, sodium citrate, anhydrous citric acid, sucralose, and sodium benzoate evenly, add solution A dropwise to it while mixing, and disperse evenly with a grinding rod. Pass the obtained sample through a 20-mesh sieve, crush it, and dry it at 60°C for 2 hours to obtain diluted granules.
[0078] 2. Preparation of dry suspension:
[0079] Sucrose, starch, xanthan gum, talcum powder, strawberry essence, silicon dioxide are mixed uniformly to obtain mixture B, and the diluted gr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com