Dexibuprofen sustained-release tablet and preparation process thereof
A sustained-release tablet and process technology, applied in the field of dextro-ibuprofen sustained-release tablet and its preparation, can solve the problems such as the inability to improve the sticking and punching phenomenon of tablet pressing, lack of anti-sticking measures, etc. Good performance and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The dry granulation of embodiment 1 Dexibuprofen
[0054] (1) See Table 1 for dry granulation prescription.
[0055] Table 1. Dry granulation prescription (mg / tablet)
[0056] Raw materials
Prescription 1
Prescription 2
Dexibuprofen
300 (average particle size 70μm)
300 (average particle size 30μm)
Microcrystalline Cellulose 102
45
45
[0057] colloidal silica
45
45
[0058] (2) Preparation method
[0059] Mix Dexibuprofen, microcrystalline cellulose 102 and colloidal silicon dioxide, pass through a 26-mesh sieve, and then use a high-speed rotary tablet press (GZPK3045 high-speed rotary tablet press, 45 punches, Shanghai Tianxiang Jiantai Pharmaceutical Co., Ltd. Co., Ltd.) pressed flakes, and the flakes passed through a swing granulator (26 mesh screen) to obtain dry granules. See the process flow figure 1 .
[0060] (3) Results
[0061] There is no sticking and punching phenomenon in ...
Embodiment 2
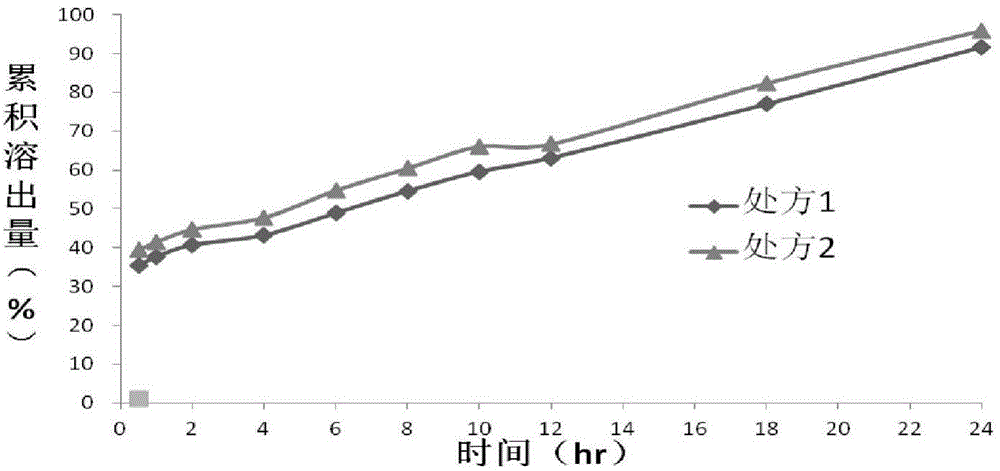
[0062] Embodiment 2 Preparation and dissolution behavior of Dexibuprofen sustained-release tablets of the present invention
[0063] The dry-process granules prepared by the two prescriptions in Example 1 were pressed into tablets respectively according to the following prescriptions, and the dissolution behavior of the Dexibuprofen sustained-release tablets prepared by raw materials with different particle sizes was investigated by dissolution tests.
[0064] (1) For the prescription of Dexibuprofen Sustained-release Tablets, see Table 2.
[0065] Table 2. Prescription of Dexibuprofen Sustained-release Tablets (mg / tablet)
[0066]
[0067]
[0068] (2) Preparation method
[0069] Weigh the dry-process granules obtained in Example 1 and other auxiliary materials according to the prescription of the immediate-release layer, and mix them uniformly; then weigh the dry-process granules obtained in Example 1 and other auxiliary materials according to the prescription of the ...
Embodiment 3
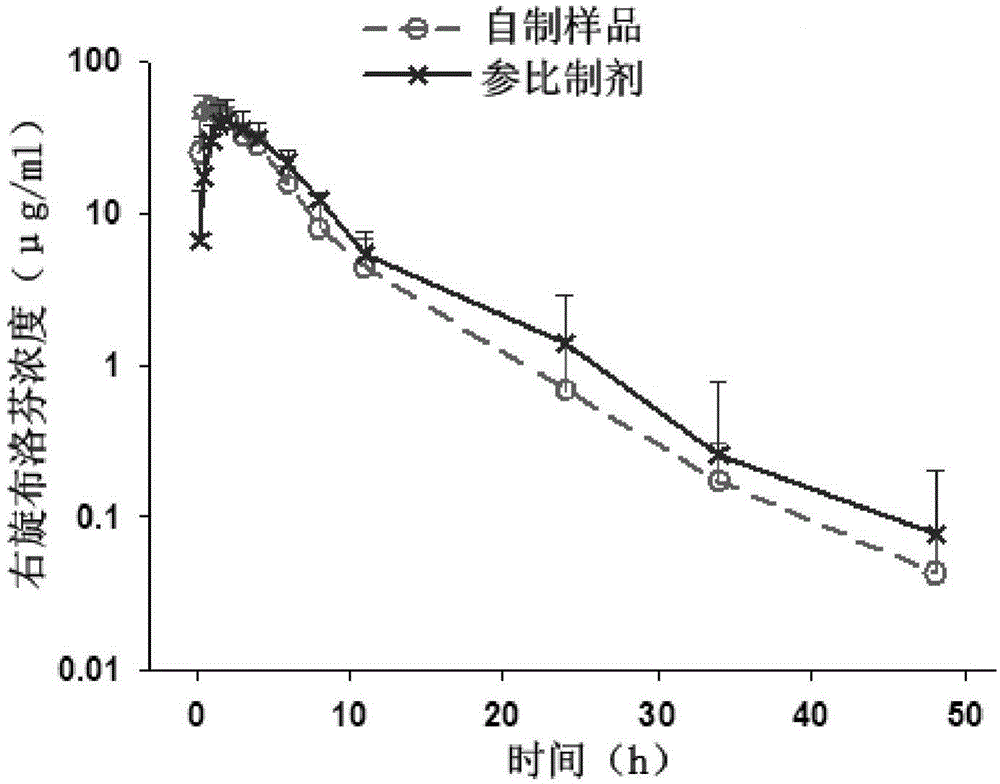
[0083] Embodiment 3 Dissolution behavior of Dexibuprofen sustained-release tablets of the present invention in different dissolution media
[0084] With the dry-process granules prepared by prescription 2 in Example 1, according to the prescription of sustained-release tablets described in Example 2, scale-up production of Dexibuprofen sustained-release tablets of the present invention (hereinafter referred to as "self-made samples") in a workshop , compared with the similar product (trade name Maxibupen ER, specification 300mg, batch number 11003, manufacturer: Korea Hanmi Pharmaceutical Co., Ltd.; hereinafter referred to as "reference preparation") in different dissolution media Research:
[0085] Dissolution medium:
[0086] 1. pH 1.0 hydrochloric acid solution: 9ml hydrochloric acid, dilute with water to 1000ml, shake well, and you get it;
[0087] 2. Phosphate buffer solution of pH 6.8: 6.8g potassium dihydrogen phosphate and 0.9583g sodium hydroxide, add water to disso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Medium volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com