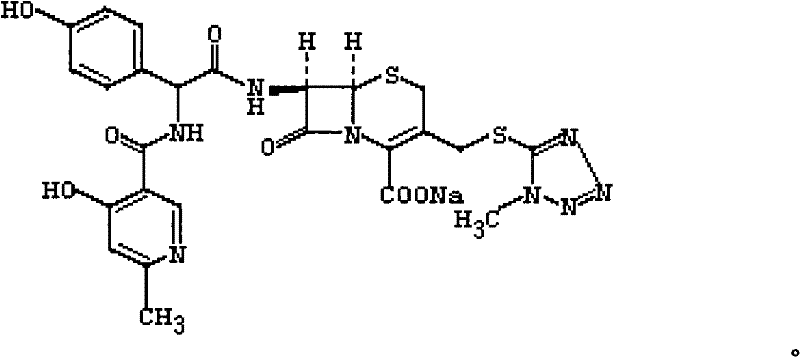
Cefpiramide sodium powder injection and preparation method thereof
A technology of cefpiramide sodium powder and cefpiramide sodium, which is applied in the field of pharmaceutical preparations, can solve the problems of high content of related substances in powder injections and damage to the structure of cefpiramide sodium, so as to avoid high content of related substances, plump appearance and high quality. controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
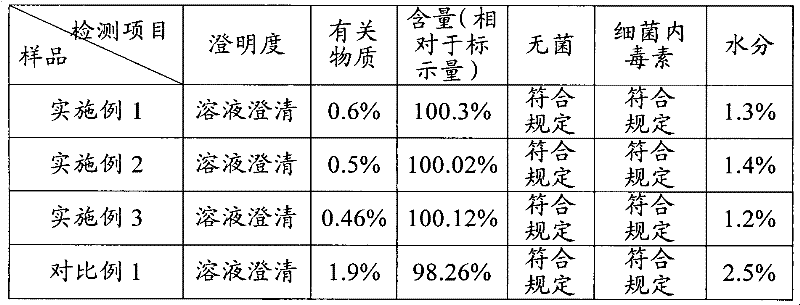
Embodiment 1
[0043] 1. Prescription composition
[0044] Cefpiramide sodium 518g (equivalent to cefpiramide 500g)
[0045] Sodium Benzoate 37.5g
[0046] Add water for injection to 2600g
[0047] 5% sodium bicarbonate aqueous solution appropriate amount
[0048] Packed into 1000 bottles
[0049] 2. Preparation method (specification: 0.5g)
[0050] Dissolve the cefpiramide sodium and sodium benzoate of the prescribed amount in water for injection accounting for 80% of the total mass of water for injection, stir until completely dissolved, add water for injection to 2600g to form a medicinal liquid, and then dropwise add a mass of 100% to the above medicinal liquid. Divide concentration into 5% sodium bicarbonate aqueous solution, adjust the pH value of the medicinal solution to be 6.6, obtain a medicinal solution with a pH value of 6.6; add 7.8g gac and stir for 30 minutes in the medicinal solution with a pH value of 6.6, and coarsely filter with filter paper Afterwards, use a 0.22 μm ...
Embodiment 2
[0052] 1. Prescription composition
[0053] Cefpiramide sodium 1036g (equivalent to cefpiramide 1000g)
[0054] Sodium Benzoate 75g
[0055] Add water for injection to 5200g
[0056] 5% sodium bicarbonate aqueous solution appropriate amount
[0057] Packed into 1000 bottles
[0058] 2 Preparation method (specification: 1.0g)
[0059] Dissolve the prescribed amount of cefpiramide sodium and sodium benzoate in water for injection accounting for 80% of the total mass of water for injection, stir until completely dissolved, add water for injection to 5200g to form a medicinal liquid, and then dropwise add a mass of 100% to the above medicinal liquid. Concentration is 5% sodium bicarbonate aqueous solution, the pH value of adjusting medicinal liquid is 6.7 and obtains the medicinal liquid that pH value is 6.7; In the medicinal liquid that pH value is 6.7, add 15.6g gac and stir for 30 minutes, after coarse filtration with filter paper Use a 0.22 μm microporous membrane to ster...
Embodiment 3
[0061] 1. Prescription composition
[0062] Cefpiramide sodium 2072g (equivalent to cefpiramide 2000g)
[0063] Sodium Benzoate 150g
[0064] Add water for injection to 10400g
[0065] 5% hydrochloric acid aqueous solution appropriate amount
[0066] Packed into 1000 bottles
[0067] 2. Preparation method (specification: 2.0g)
[0068] Dissolve the prescribed amount of cefpiramide sodium and sodium benzoate in water for injection accounting for 80% of the total mass of water for injection, stir until completely dissolved, add water for injection to 10400g to form a medicinal liquid, and then dropwise add a mass of 100% to the above medicinal liquid. Concentration is 5% hydrochloric acid aqueous solution, and the pH value of adjusting medicinal liquid is 6.5 and obtains the medicinal liquid that pH is 6.5; In the medicinal liquid that pH value is 6.5, add 31.2g gac and stir for 30 minutes, filter with filter paper and use 0.22 μm microporous membrane sterilizing filtration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com