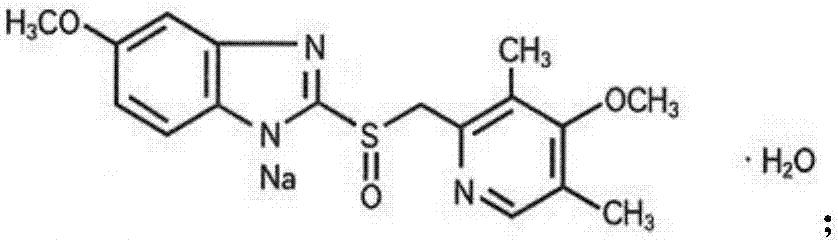
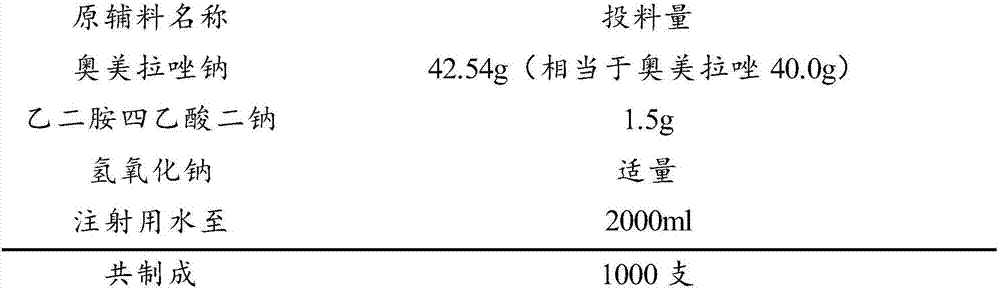
Omeprazole sodium freeze-dried powder injection preparation method
A technology of omeprazole sodium and freeze-dried powder injection, which is applied in the field of preparation of omeprazole sodium freeze-dried powder injection, can solve problems such as polymerization reaction, influence on product quality, discoloration, etc., to ensure quality and improve drug use Effects of safety, quality improvement and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of omeprazole sodium freeze-dried powder injection, comprises the following steps:
[0025] A) mixing the metal ion complexing agent with part of the water for injection, adjusting the pH value to 9.5-11 to obtain the first mixed solution;
[0026] B) mixing omeprazole sodium with the first mixed solution, adjusting the pH value to 9.9-11.1;
[0027] C) supplementing remaining water for injection to obtain omeprazole sodium solution;
[0028] D) Prefreeze the omeprazole sodium solution for 2 to 3 hours, and the prefreezing temperature is -35 to -40°C;
[0029] E) After pre-freezing, heat up to 0°C at a speed of 7-9°C / h, then rise from 0°C to 30°C at a speed of 3-5°C / h, and then increase the temperature from 0°C to 30°C at a speed of 1-3°C / h Raise the temperature from 30°C to 35°C-40°C to obtain omeprazole sodium powder;
[0030] F) keeping the omeprazole sodium powder at 35-40° C. for 3-5 hours to obtain omeprazole ...
Embodiment 1
[0054]
[0055] (1) During the entire preparation and filling process, the whole process should be protected from light.
[0056] Add 1200ml of water for injection into the concentrated irrigation, control the temperature at about 10°C, add 1.5g of EDTA, stir well to contact with the tank wall, and adjust the pH to 10.6 with 0.1M NaOH solution.
[0057] (2) Add 42.54g of omeprazole sodium (dried and pure) into the concentrated preparation tank, and stir to dissolve. Use 0.1M NaOH solution to adjust the pH to 10.8, add 1g of activated carbon, stir for 15 minutes, and decarbonize it with a titanium rod into a dilute tank.
[0058] (3) Add water for injection to 2000ml in the dilute preparation tank. After the semi-finished product passes the test, use a 0.45μm microporous filter element for coarse filtration, and finally pass a 0.22μm microporous filter element for fine filtration before filling and half stoppering.
[0059] Use the following process to freeze-dry:
[0060]...
Embodiment 2
[0065]
[0066]
[0067] (1) During the entire preparation and filling process, the whole process should be protected from light.
[0068] Add 1200ml of water for injection into the concentrated irrigation, control the temperature at about 10°C, add 1.5g of EDTA, stir well to contact with the tank wall, and adjust the pH to 10.7 with 0.1M NaOH solution.
[0069] (2) Add 42.54g of omeprazole sodium (dried and pure) into the concentrated preparation tank, and stir to dissolve. Use 0.1M NaOH solution to adjust the pH to 10.8, add 1g of activated carbon, stir for 15 minutes, and decarbonize it with a titanium rod into a dilute tank.
[0070] (3) Add water for injection to 2000ml in the dilute preparation tank. After the semi-finished product passes the test, use a 0.45μm microporous filter element for coarse filtration, and finally pass a 0.22μm microporous filter element for fine filtration before filling and half stoppering.
[0071] The freeze-drying process is:
[0072...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com