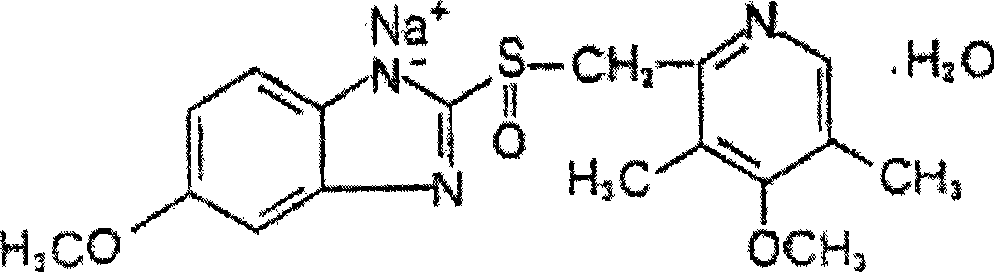
Omeprazole sodium compound and method for synthesizing the same
A technology of omeprazole sodium and a synthetic method, which is applied in the field of medicine, can solve problems such as low yield, poor purity, and long reaction time, and achieve the effects of good product purity, cost reduction, and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole
[0038] 180 grams (1mol) of 2-mercapto-5-methoxy-1H-benzimidazole and 222 grams (1mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridine hydrochloride Add 5 liters of ethanol and acetone mixed solvent (V:V=3:1), comprise the sodium hydroxide of 80 grams (2mol), add 5 grams (0.033mol) sodium iodide simultaneously, this reaction mixture is heated and refluxed 1.5 hours , cooled to room temperature, filtered to remove insoluble matter, the filtrate was distilled off under reduced pressure to remove most of the solvent, the residue was dissolved in 3 liters of ethyl acetate, washed twice with 1 liter of water, the organic phase was dried with anhydrous sodium sulfate, filtered, concentrated , add 400ml of acetone to the residue, freeze the organic phase to 0°C, precipitate a solid overnight, filter to obtain 315.8 grams of off-white solid 5-methoxy-2-[(4-methoxy-3,5-lutidine -2-yl)methy...
Embodiment 2
[0039] The synthesis of embodiment 2 omeprazole
[0040]5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole prepared in Example 1 was added to 1500ml of Ethyl acetate, the reaction system is cooled to -25 ° C, maintain this reaction temperature, slowly add 40ml of peracetic acid, continue to react for 0.5 hours after adding, adjust the pH value of the reaction system with 10% sodium hydroxide at the same time, maintain at When pH=8, a precipitate precipitated out, filtered, washed the filter cake with water, dried, and then recrystallized with methanol to obtain 314.4 g of white solid omeprazole, yield: 94.8%.
Embodiment 3
[0041] The synthesis of embodiment 3 omeprazole sodium
[0042] The omeprazole that embodiment 2 is made joins in the isopropanol of 1000ml, adds dropwise aqueous sodium hydroxide solution (the sodium hydroxide of 37.1 grams is dissolved in the distilled water of 60ml) simultaneously, this mixture is stirred at room temperature 1 hour , then filtered, washed the filter cake with 40ml of isopropanol, and vacuum-dried at 40°C to obtain 344 grams of product, yield: 98.1%. HPLC: 99.8%.
[0043] Elemental analysis theoretical value C: 52.9%, H: 5.2%, N: 10.9%, 0: 16.6%, S: 8.3%; experimental value C: 53.1%, H: 5.3%, N: 11.0%, 0: 16.6% , S: 8.2%.
[0044] 1 H-NMR (DM SO): δ(10 -6 ): 2.09-2.20 (s, 6H, CH 3 ), 3.68-3.81 (s, 6H, OCH 3 ), 4.67-4.78 (m, 2H, CH 2 ), 7.05-7.56 (m, 3H, benzene H), 8.19 (s, 1H, pyridine H), 13.44 (s, 1H, NH).
[0045] MS (m / e): 330 (M + , 100%), 196 (M + ,-C 9 h 12 NO, 15%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com