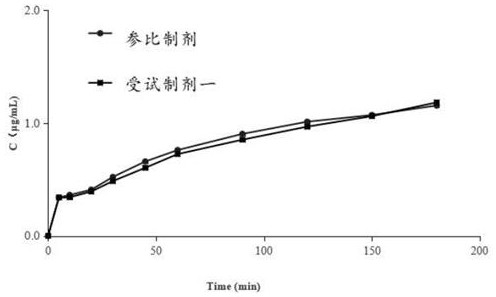
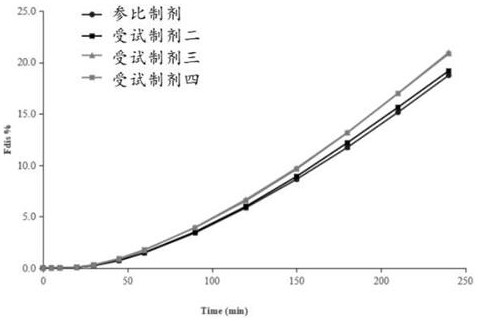
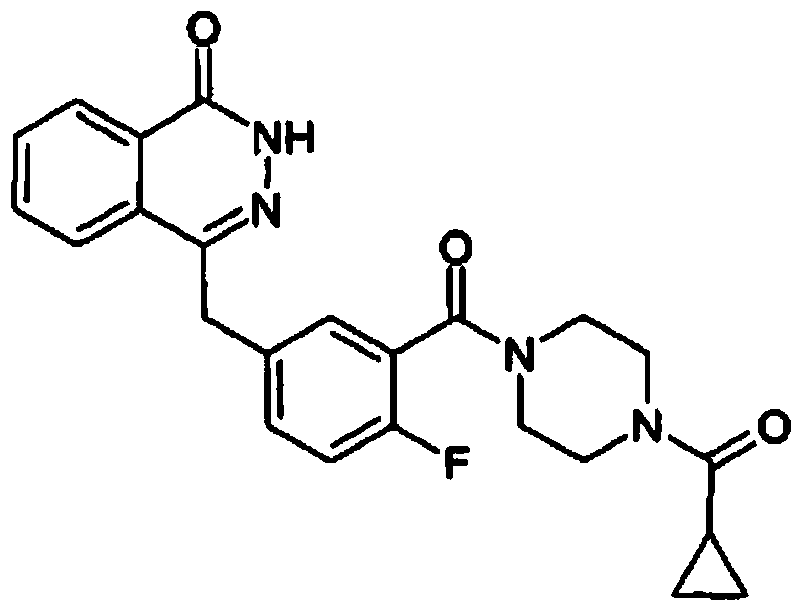
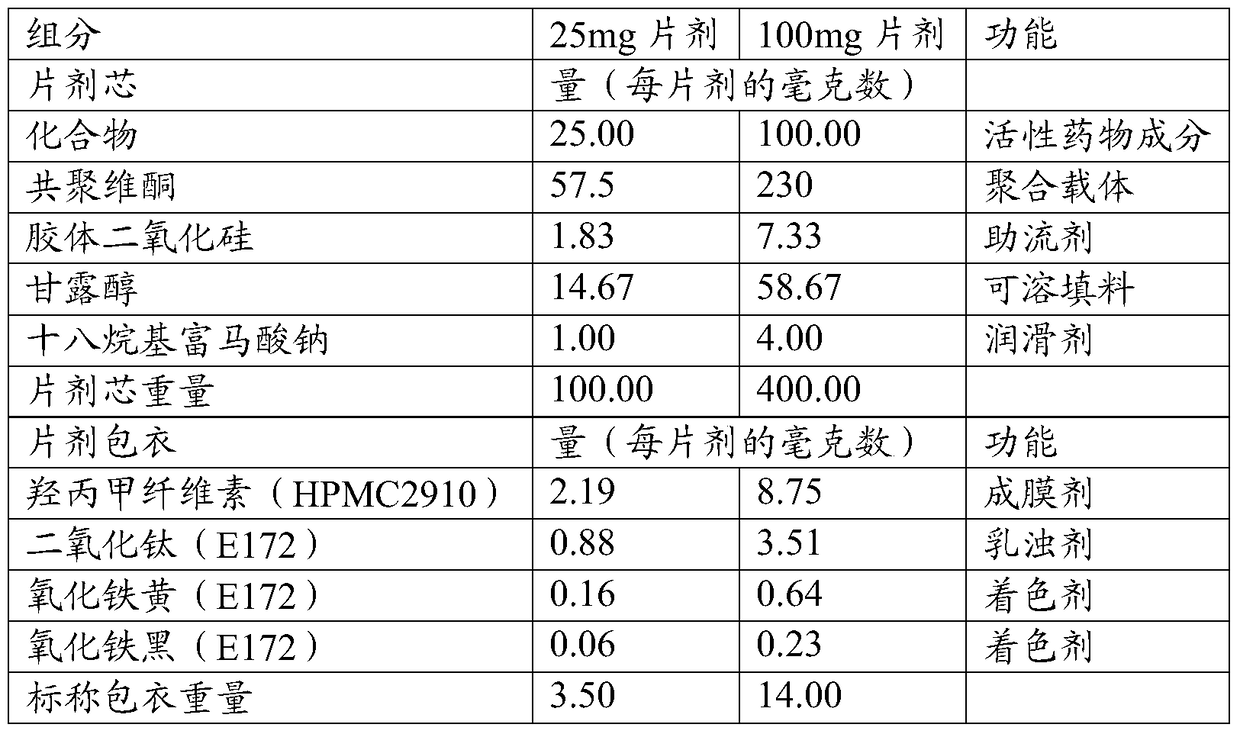
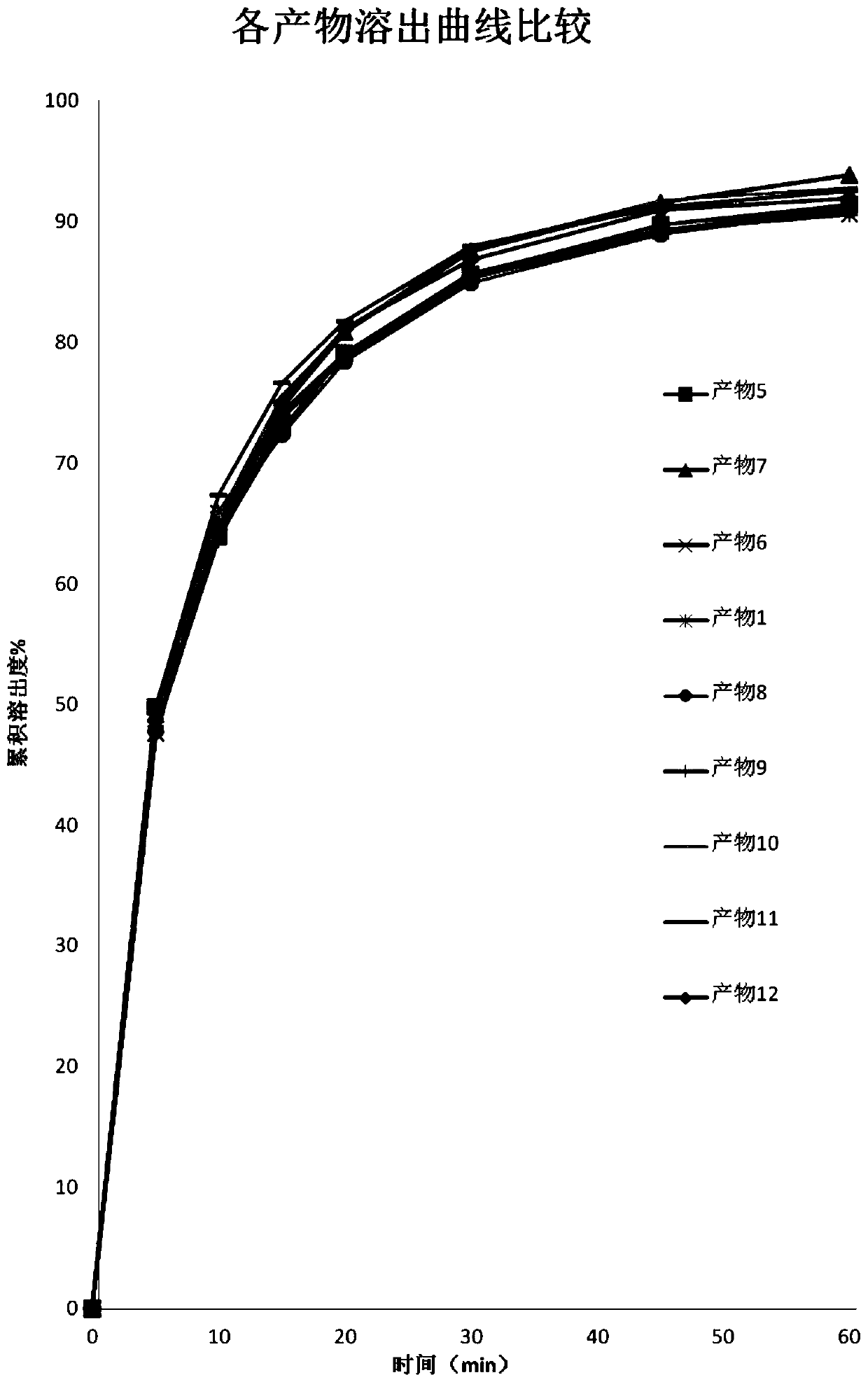
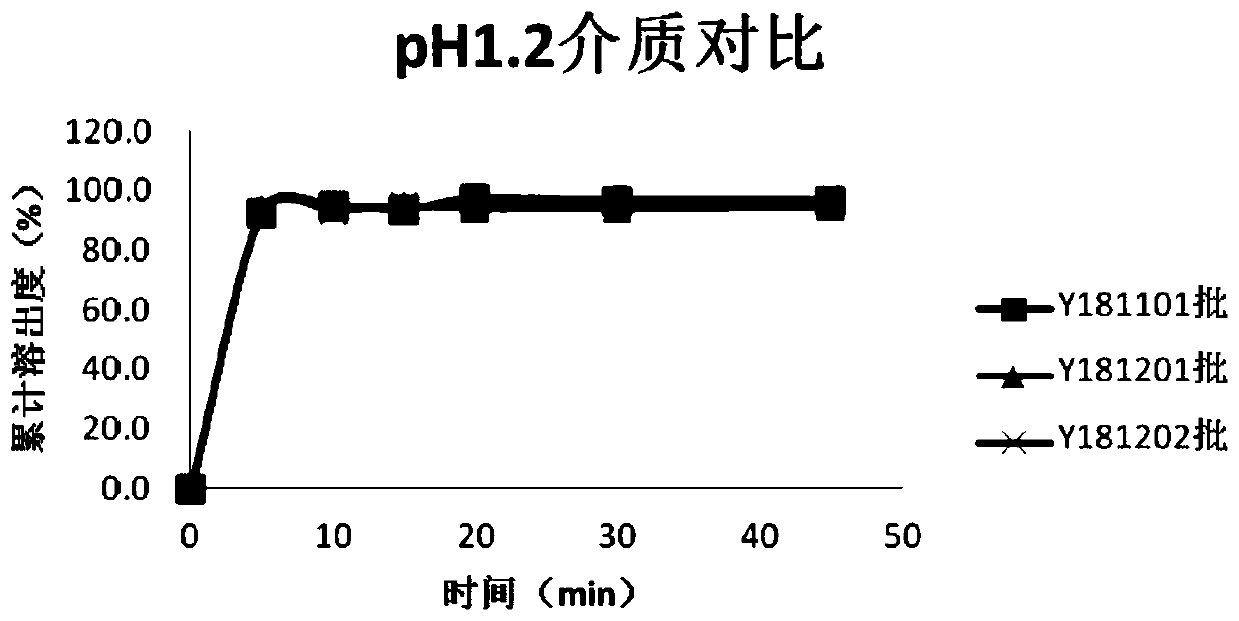
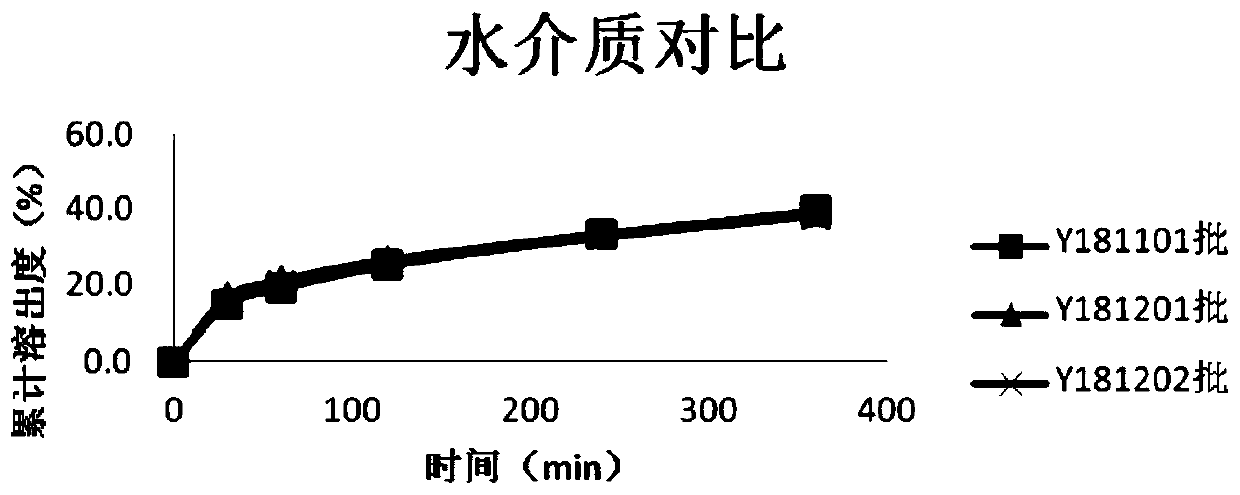
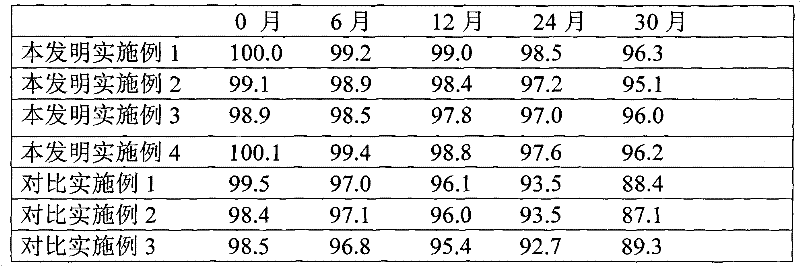
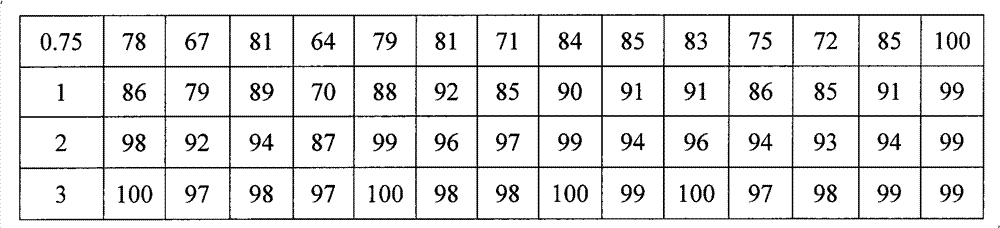
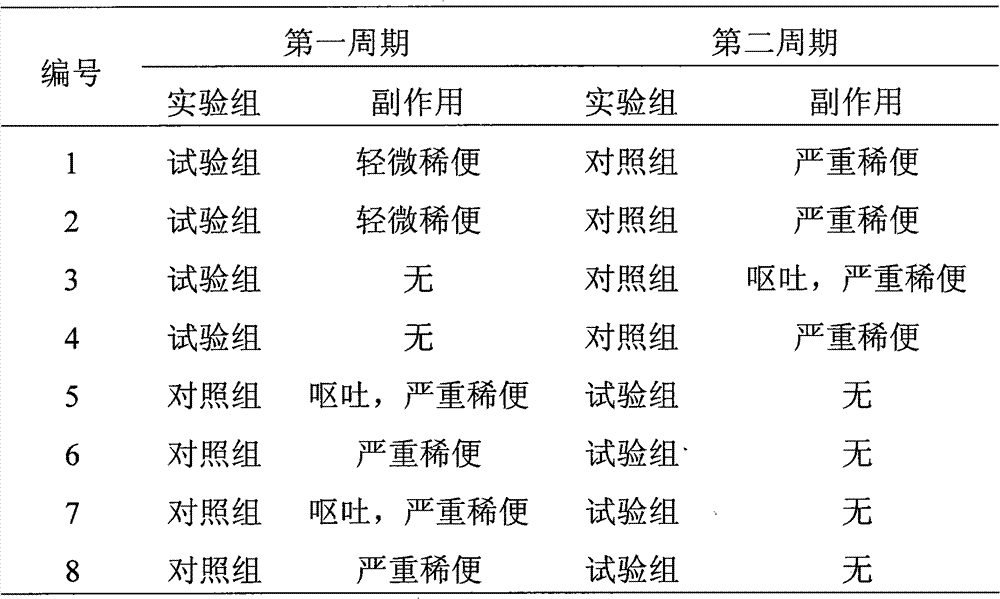
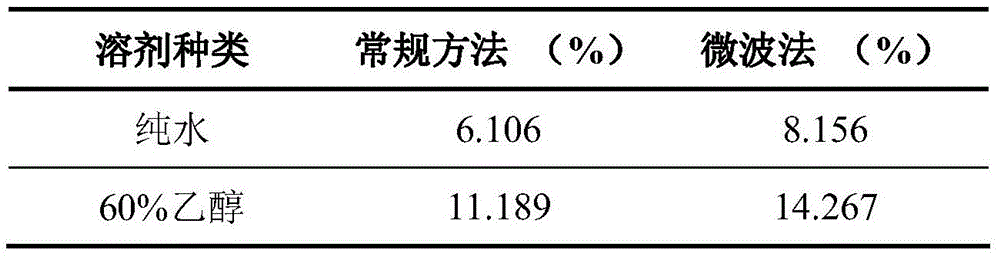
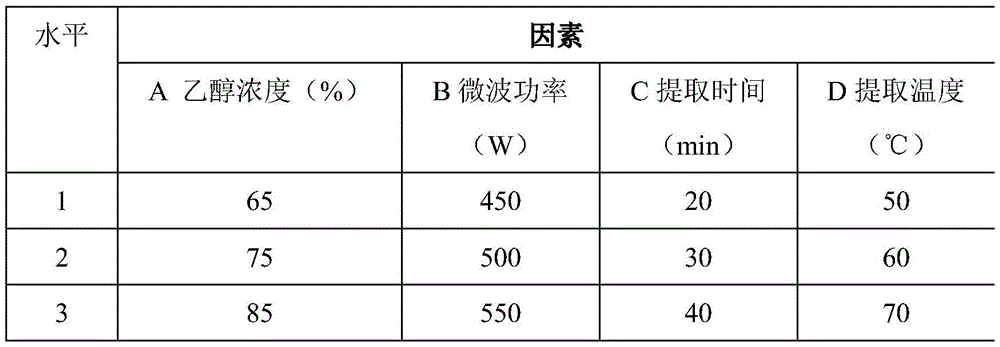
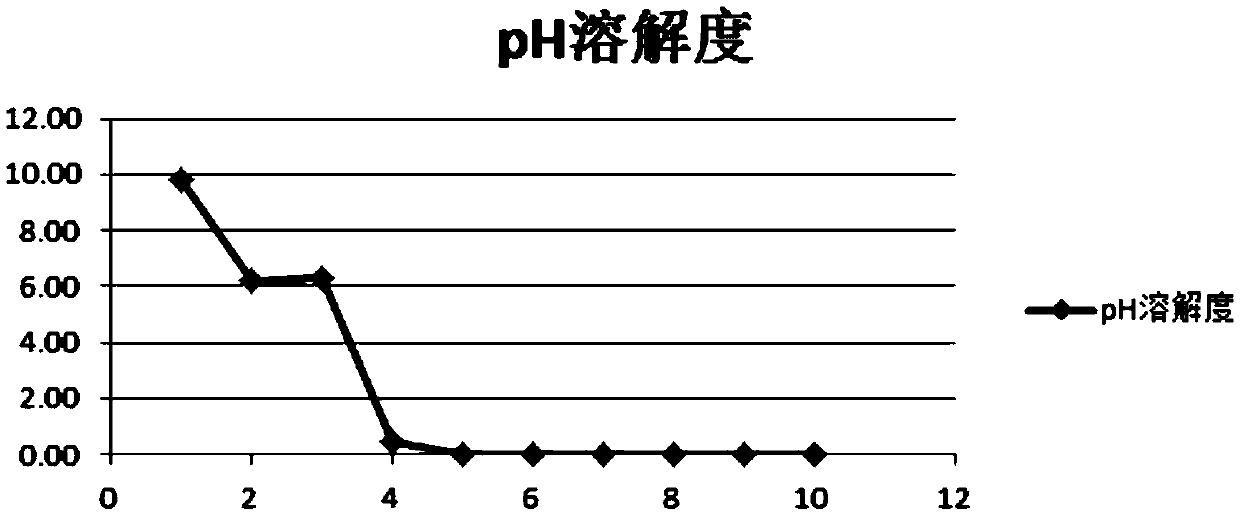
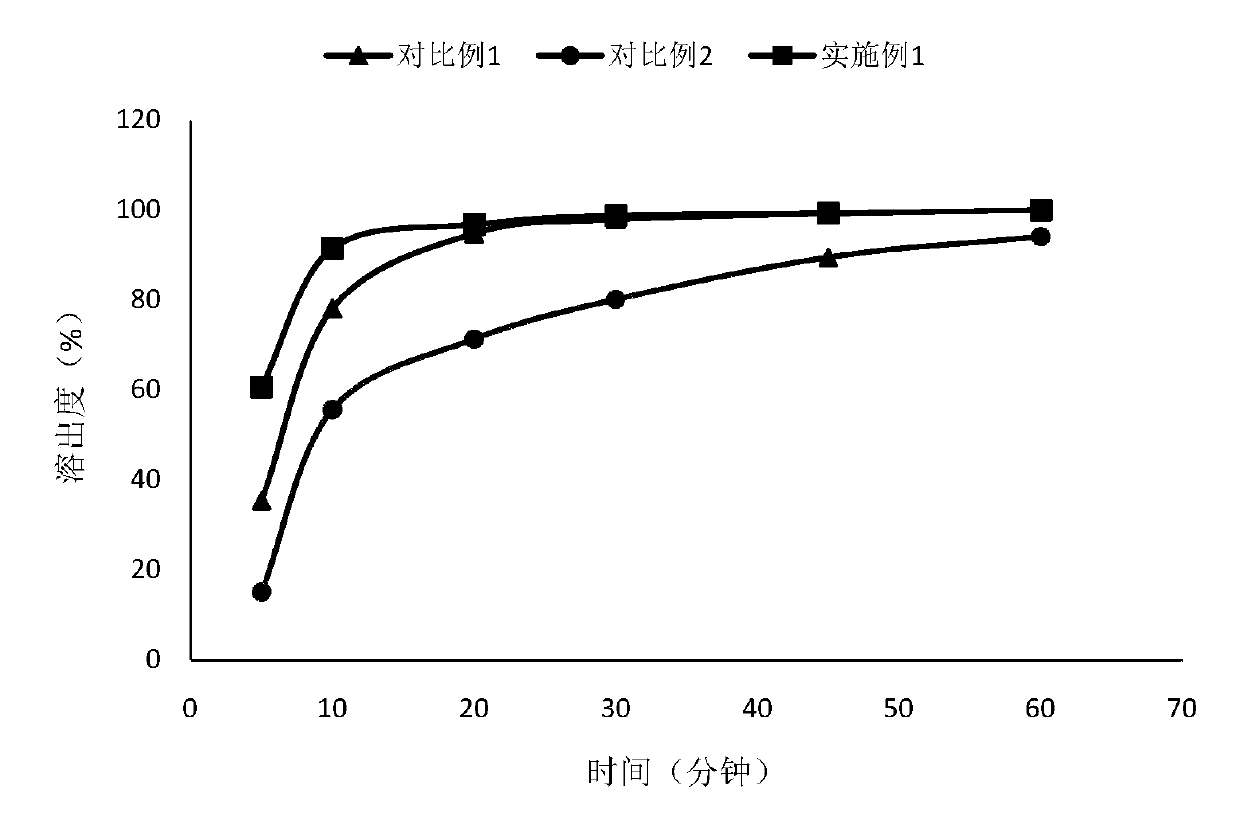
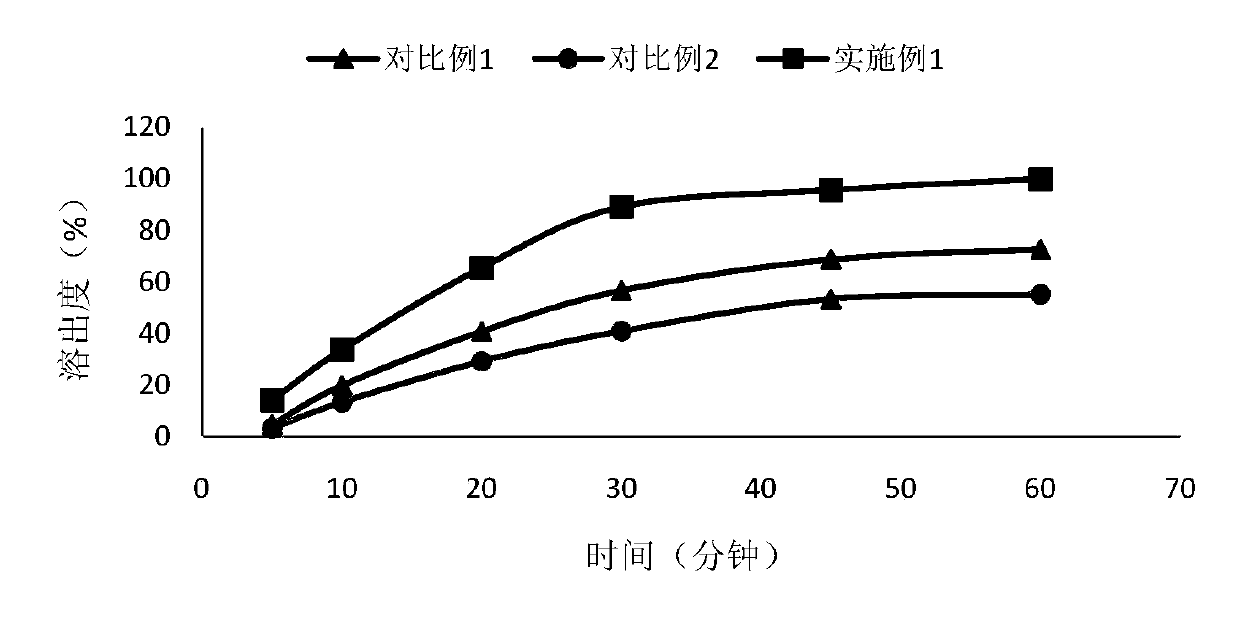
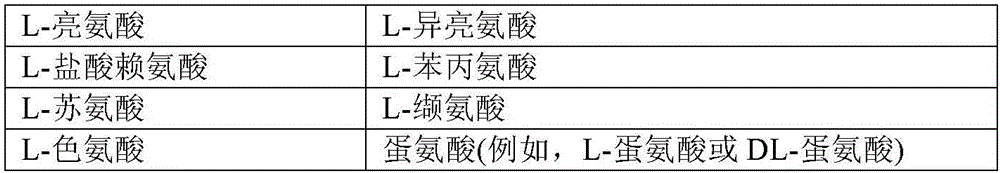
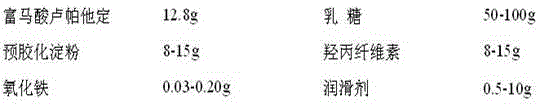Patents
Literature
51results about How to "Guaranteed bioavailability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
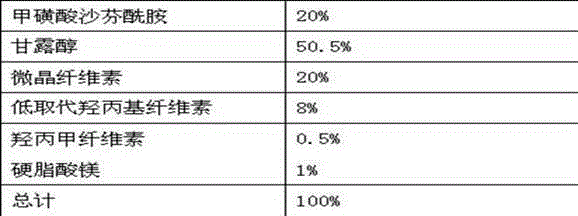
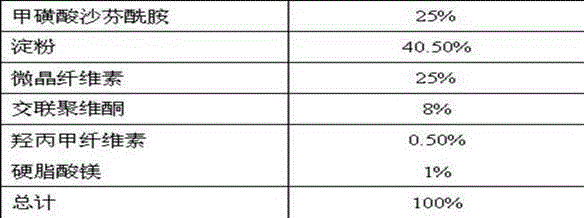
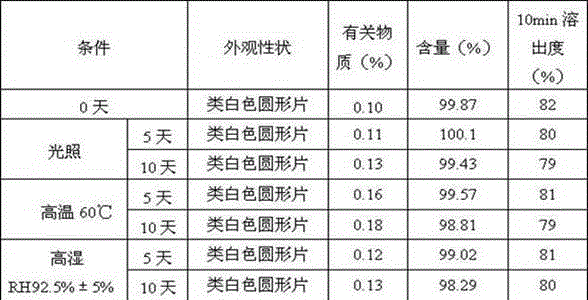
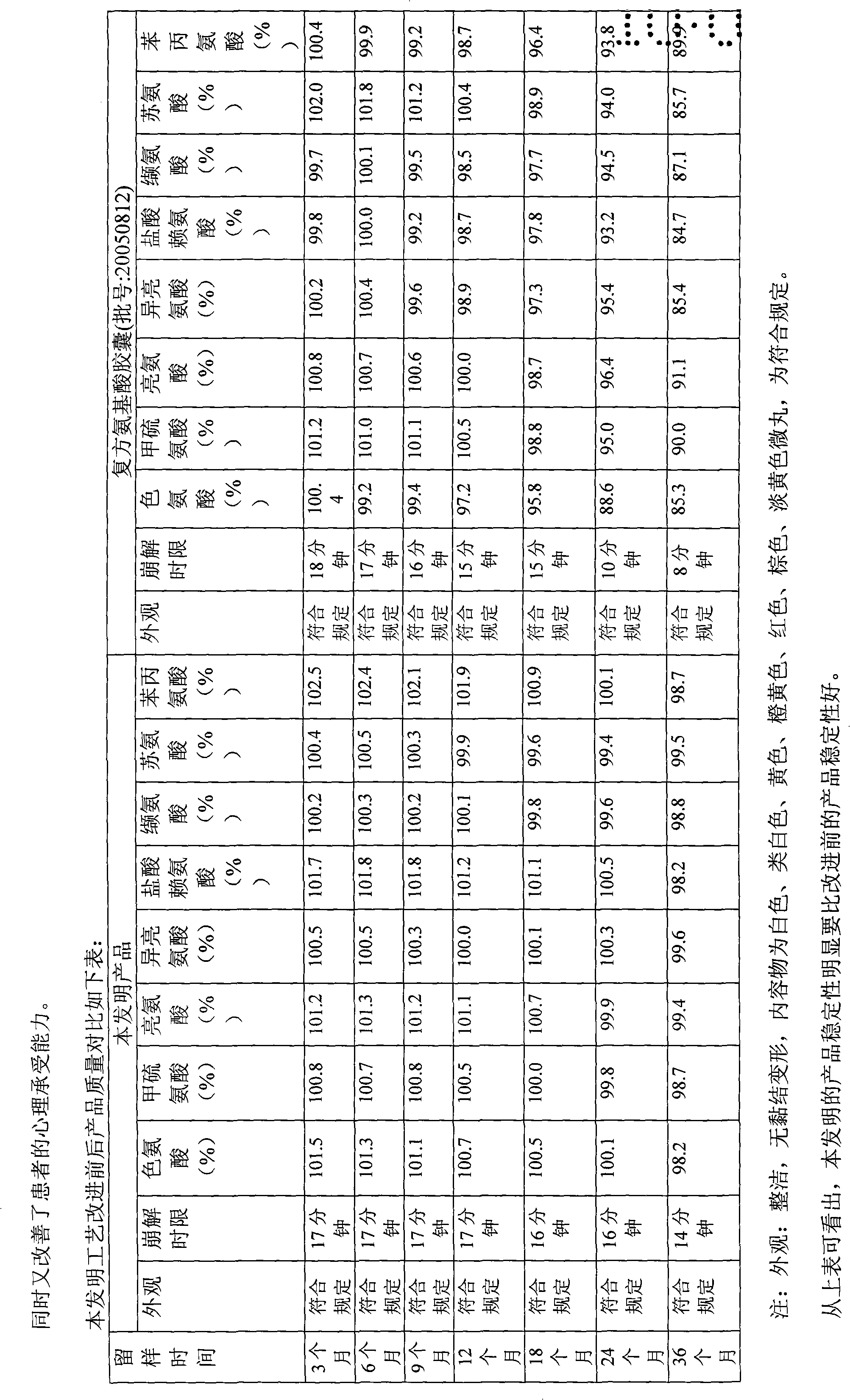
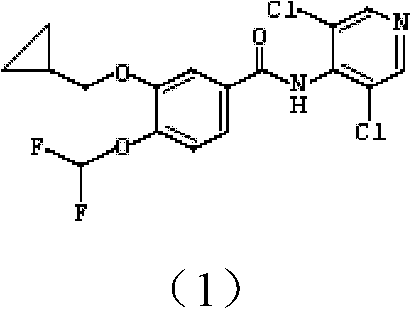
Pharmaceutical composition containing safinamide mesylate and preparation method of pharmaceutical composition
InactiveCN104546747AGuaranteed bioavailabilityEasy to operateOrganic active ingredientsNervous disorderWater solubleDissolution
The invention belongs to the technical field of medicines, and relates to a safinamide mesylate pharmaceutical composition having a good dissolution effect. The pharmaceutical composition contains safinamide mesylate, a water-soluble excipient, a disintegrant, a lubricant and the like, wherein the safinamide mesylate undergoes micronization treatment, and the grain size of 90% of the safinamide mesylate is controlled within 5-50microns, preferably within 10-20microns. The medicine prepared by the method disclosed by the invention is good in dissolution effect, so that the bioavailability of the medicine is effectively improved.
Owner:AVENTIS PHARMA HAINAN
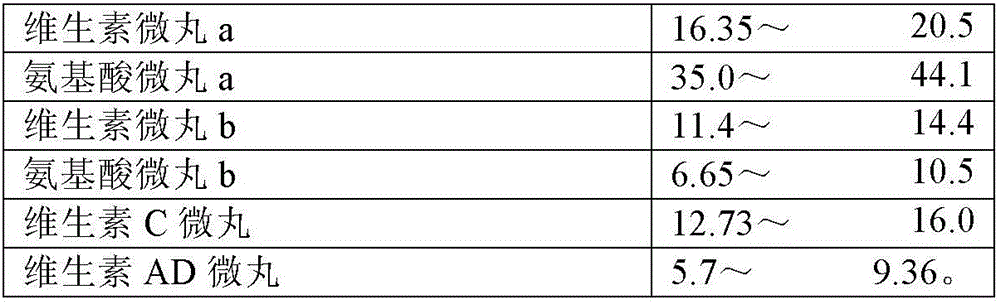
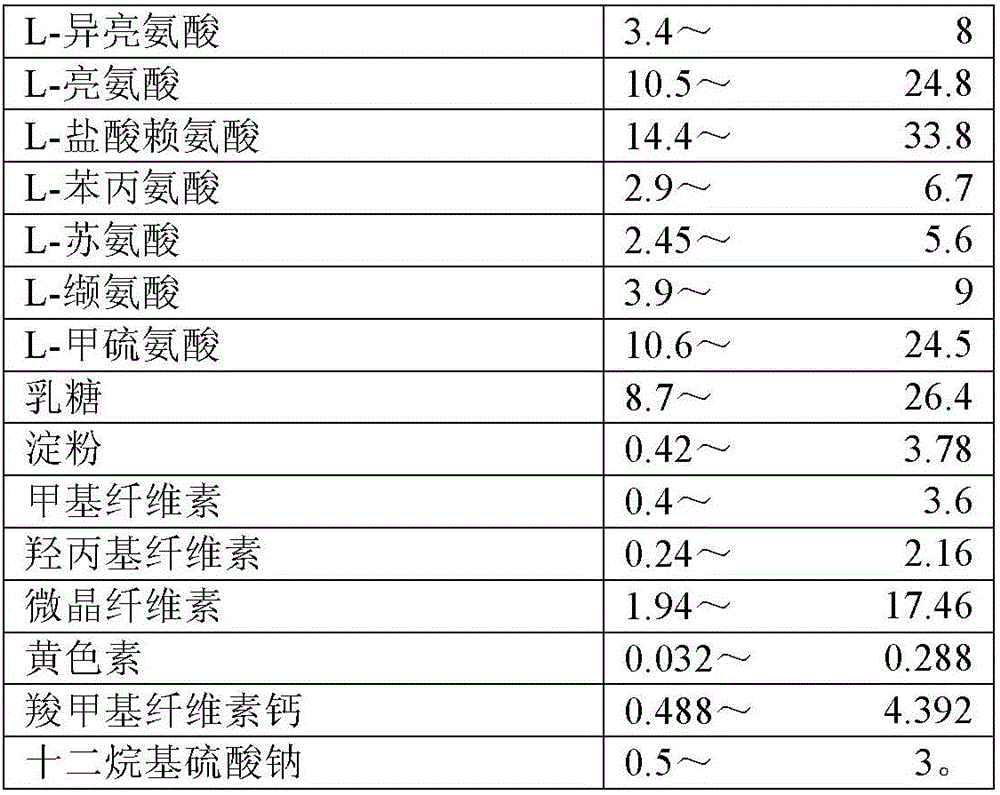
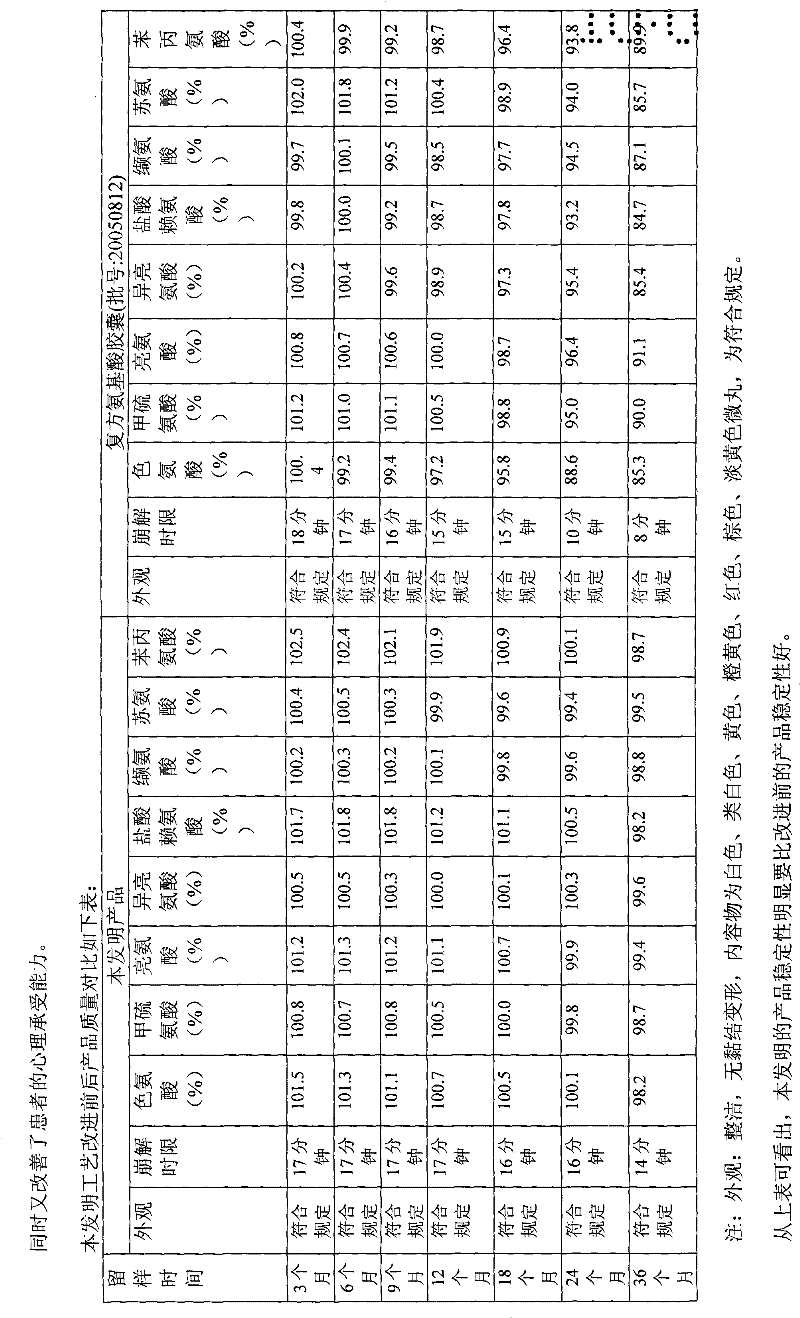
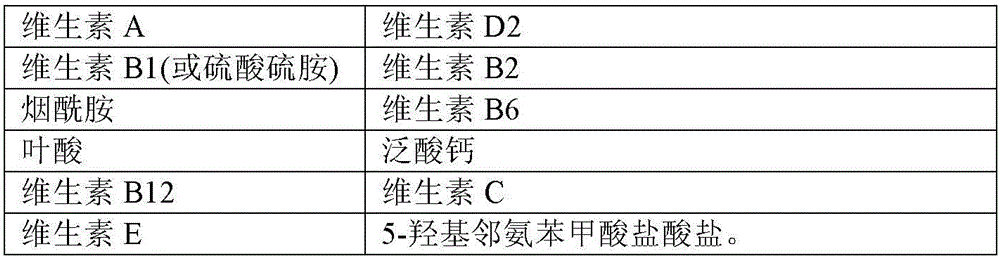
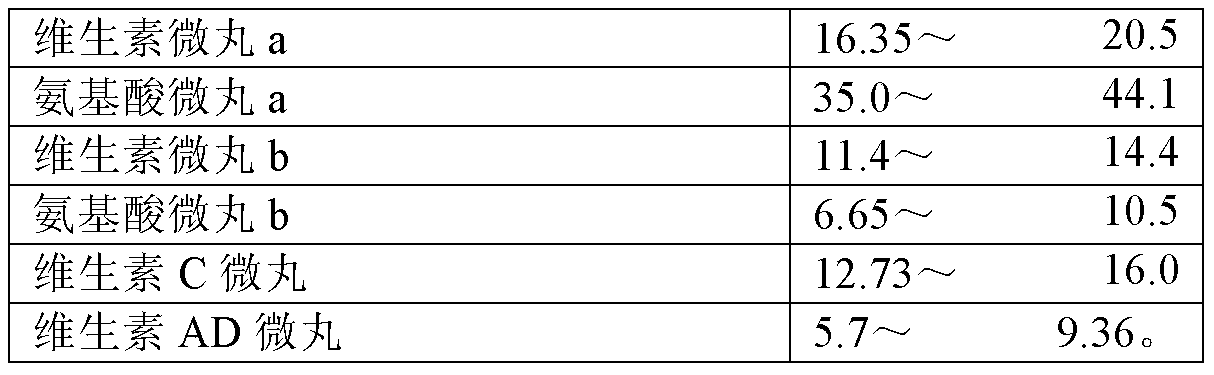
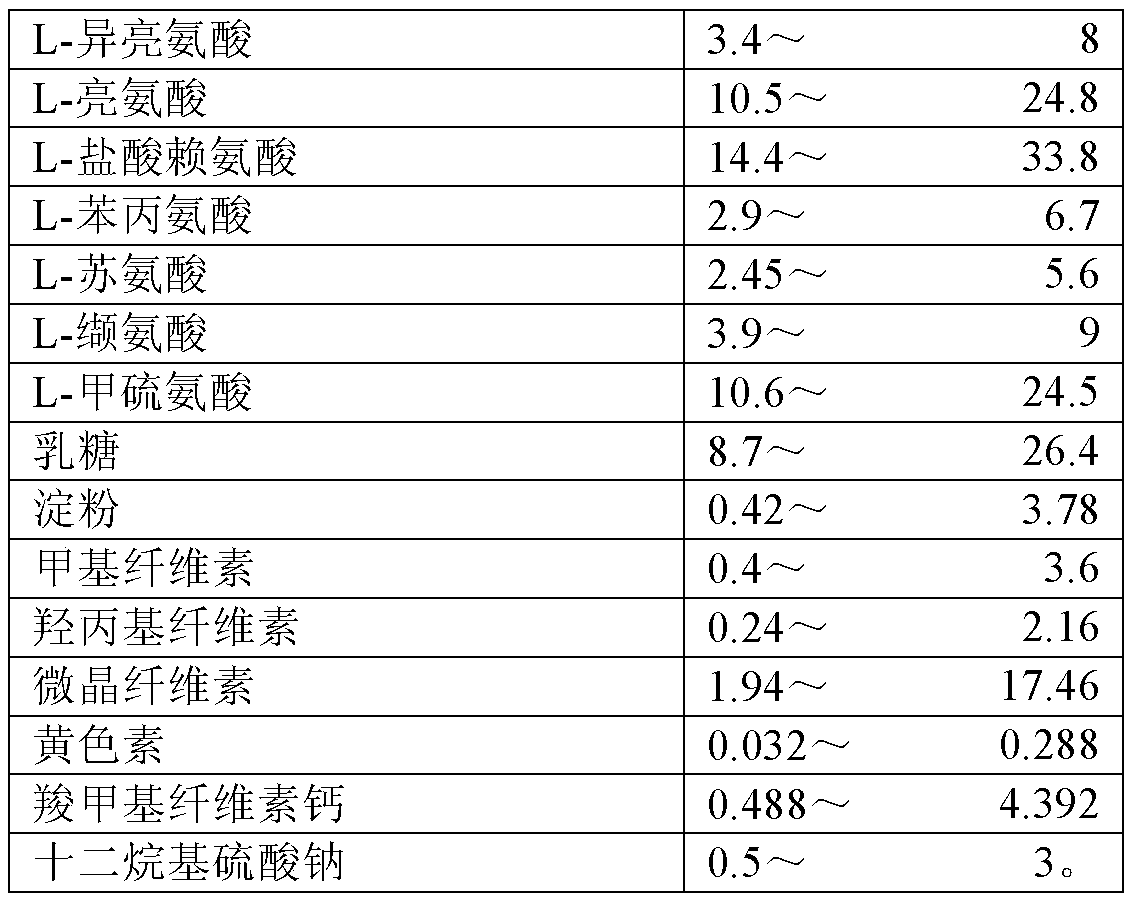
8-amino acid/11-vitamin containing micro granule capsule and preparation method thereof
ActiveCN101773512AImprove bioavailabilityUnique craftPeptide/protein ingredientsHydroxy compound active ingredientsDiseaseLiver and kidney
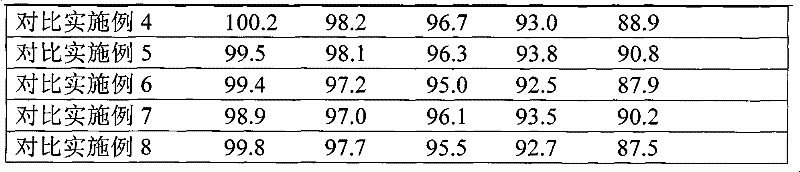
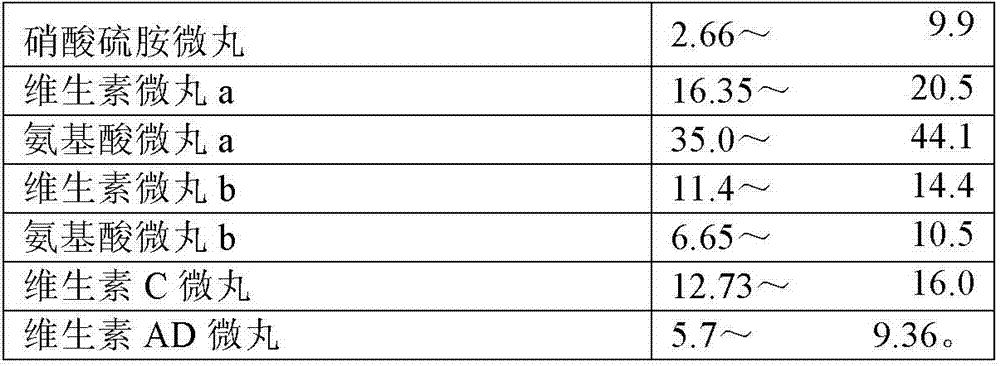
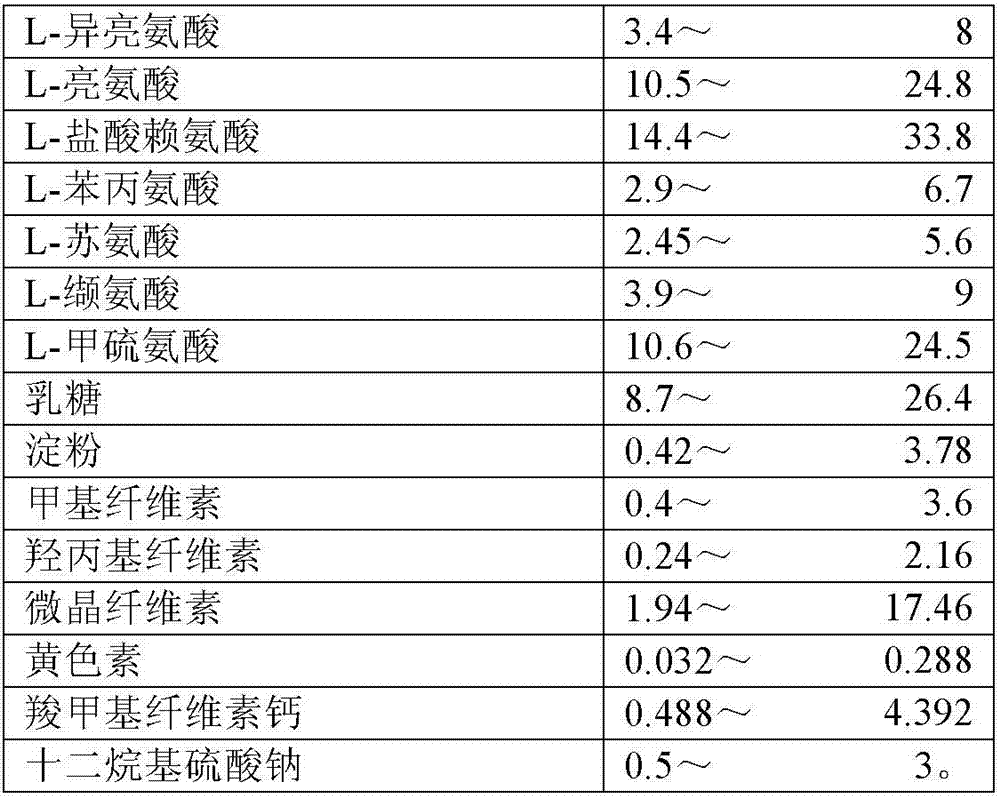
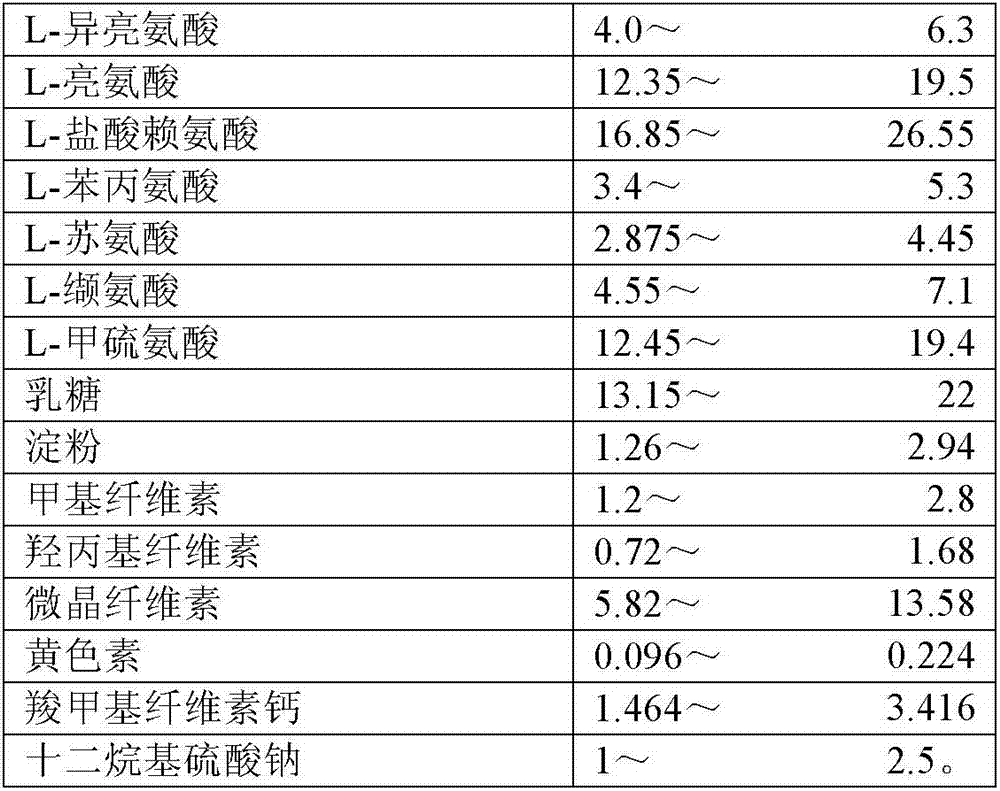
The invention discloses an 8-amino acid / 11-vitamin containing micro granule capsule and a preparation method thereof, wherein the micro granule capsule contains 7 ball micro granules consisting of the following raw materials in part by weight: 2.66-9.9 parts of thiamine mononitrate micro granule, 16.35-20.5 parts of vitamin micro granule a, 35.0-44.1 parts of amino acid micro granule a, 11.4-14.4 parts of vitamin micro granule b, 6.65-10.5 parts of amino acid micro granule b, 12.73-16.0 parts of vitamin C micro granule and 5.7-9.36 parts of vitamin AD micro bead. The method has the advantages of scientific and reasonable proportion, good stability of products, no toxicity and side effect and excellent biological effect, and can be used for the preparation of medicines treating chronic liver and kidney diseases and of food alleviating and eliminating physical fatigue caused by sports training.
Owner:SHENZHEN WANHE PHARMA
Compound amino acid capsule including eight amino acids and eleven vitamins
ActiveCN103142633AImprove bioavailabilityUnique craftHydroxy compound active ingredientsPeptide/protein ingredientsThiamineVitamin C
The invention relates to a compound amino acid capsule (8-11) and a preparation method thereof, specifically to a capsule including pellets of eight amino acids and eleven vitamins and a preparation method thereof. The capsule comprises 7 spherical pellets which are composed of the following raw materials by weight: 2.66 to 9.9 parts of a thiamine mononitrate pellet, 16.35 to 20.5 parts of a vitamin pellet a, 35.0 to 44.1 parts of an amino acid pellet a, 11.4 to 14.4 parts of a vitamin pellet b, 6.65 to 10.5 parts of an amino acid pellet b, 12. 73 to 16.0 parts of a vitamin C pellet and 5.7 to 9.36 parts of a vitamin AD bead. The capsule provided by the invention has the advantages of a scientific and reasonable ratio, good stability, no toxic and side effects, high security and good biological effects and can be applied in preparation of medicines used for treating chronic liver and kidney diseases and of foodstuffs used for alleviating and eliminating physical fatigue caused by exercise training. The capsule provided by the invention has characteristics superior to those of the prior art.
Owner:SHENZHEN WANHE PHARMA
Pharmaceutical composition containing roflumilast
InactiveCN102626410ASimple process operationGuaranteed bioavailabilityAntipyreticAnalgesicsWater soluble polymersAqueous solubility
The invention relates to a pharmaceutical composition containing roflumilast. The pharmaceutical composition containing roflumilast has good dissolution effects and comprises components of roflumilast, water-soluble excipients and water-soluble polymer adhesives, wherein roflumilast is subjected to micronization so that 90% or more by volume of roflumilast particles have particle sizes of 5 to 50 microns and preferably have particle sizes of 10 to 20 microns. The pharmaceutical composition containing roflumilast has good dissolution effects so that the bioavailability of the pharmaceutical composition containing roflumilast is improved effectively.
Owner:BEIJING WANQUAN SUNSHINE MEDICAL TECH CO LTD
Bilastine-containing pharmaceutical composition and preparation method thereof
InactiveCN103356616AGuaranteed bioavailabilityEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsWater solubleBioavailability
The invention belongs to the technical field of medicines, and relates to a bilastine pharmaceutical composition with good dissolution effect. The pharmaceutical composition comprises bilastine, a water-soluble excipient, a disintegrating agent, a lubricating agent and the like, wherein the bilastine is micronized, the grain size of 90% of the bilastine is controlled to 5 to 50 microns and is specifically preferable to 10 to 20 microns. A medicine prepared by the preparation method shows good dissolution effect, and therefore, the bioavailability of the medicine can be effectively improved.
Owner:BEIJING VENTUREPHARM BIOTECH
Stable cefeclor dispersible tablet and preparation method thereof
ActiveCN101912373AImprove the lubrication effectLittle difference in tablet weightAntibacterial agentsOrganic active ingredientsCross-linkHypromellose
The invention belongs to the technical field of medicament preparations, and particularly relates to a cefeclor dispersible tablet which is characterized by being prepared from the following components in parts by weight: 25g of cefeclor, 11.5g of microcrystalline cellulose, 5-7g of hydroxypropyl cellulose, a proper amount of 2-percent hydroxypropyl methylcellulose 40-percent ethanol solution, 5g of pregelatinized starch, 2.8-4g of cross-linked polyvidone, 1.2g of silicon dioxide, 1-2g of magnesium laurylsulfate and 0.12g of essence. Because the magnesium laurylsulfate is used in the dispersible tablet, the preparation stability is markedly improved, the content is basically not changed after long-time placement, and the service time of the medicament is prolonged.
Owner:BEIJING JINGFENG PHARMA GRP
Taxus chinensis var mairei Chinese herbal material as well as processing method and application thereof
InactiveCN102641308AIncrease picking volumeEasy to identifyAlcoholic beverage preparationAntineoplastic agentsTaxus wallichiana var. maireiMedicinal herbs
The invention relates to a taxus chinensis var mairei Chinese herbal material, as well as a processing method and application thereof and belongs to the technical field of Chinese herbal medicine processing. The processing method of the taxus chinensis var mairei Chinese herbal material comprises the following steps of: picking new branches of the selected manually-planted taxus chinensis var mairei with the age of more than 3 years from June to August annually, wherein the lengths of the picked branches range from 5cm to 8cm; smashing the picked branches to 8-10mm, and then drying the smashed branches for 20 minutes to 1 hour till the water content of the smashed branches reach 9% to 11%, wherein the temperature of drying gas flow ranges from 200 DEG C to 250 DEG C, and the gas flow rate ranges from 6000m3 / h to 11000m3 / h; and packaging the dried branches on an automatic packaging machine to obtain the aluminium-foil-inner-packaged taxus chinensis var mairei Chinese herbal material. The taxus chinensis var mairei Chinese herbal material obtained by the processing method can be taken as medicinal slices as well as raw materials for processing medicinal liquor, and can be taken orally to treat tumors and be externally applied to treat skin cancer.
Owner:JIANGSU HONGDOUSHAN BIOLOGICAL TECH
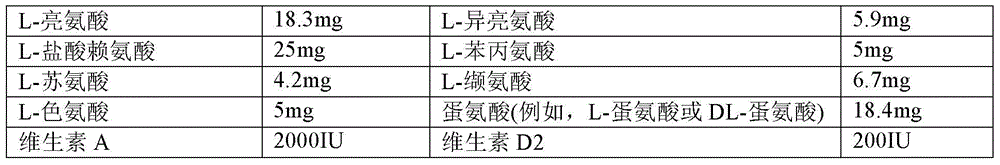
Method for preparing vitamin AD pellet, and compound amino acid capsule composition thereof
ActiveCN106821998AImprove bioavailabilityUnique craftHydroxy compound active ingredientsMetabolism disorderVitamina D2Vitamin A Alcohol
The invention relates to a method for preparing a vitamin AD pellet, and a compound amino acid capsule composition thereof. Concretely, on one hand, the invention relates to the vitamin AD pellet, which comprises vitamin A and vitamin D2; every 1g of pellet contains 2 to 200,000 I.U. of vitamin A and 0.2 to 20,000 I.U. of vitamin D2. The invention also relates to the preparation method of the vitamin AD pellet, which comprises a capsule of a compound amino acid vitamin pellet containing the vitamin AD pellet. The vitamin AD pellet and the compound amino acid vitamin capsule prepared by using the vitamin AD pellet have the characteristics of superior to existing products.
Owner:SHENZHEN WANHE PHARMA
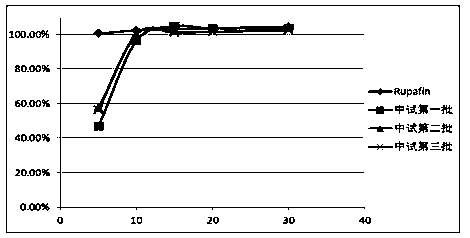
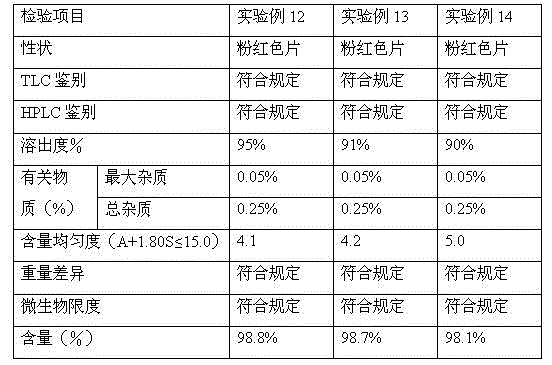
Rupatadine fumarate tablets and preparation method thereof
InactiveCN103751141AImprove discoloration, etc.Promote dissolutionOrganic active ingredientsPharmaceutical product form changePharmaceutical drugCurative effect
The invention discloses rupatadine fumarate tablets and a preparation method thereof, belonging to the technical field of medicine. The tablets are prepared by making granules from rupatadine fumarate as active ingredient, and pharmaceutical adjuvants including a filler, a binder and a disintegrant, adding a lubricant, and making into tablets. Through the screening of the type and use amount of the filler, disintegrant, binder and lubricant and the use of specific sieving mesh to sieve raw materials during a preparation process, the prepared tablets have stable properties, good content uniformity, good dissolution, small tablet weight difference and high stability, so as to ensure in vivo bioavailability and clinical curative effect.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
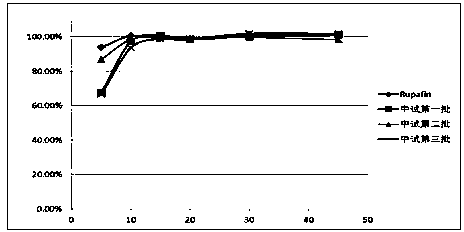
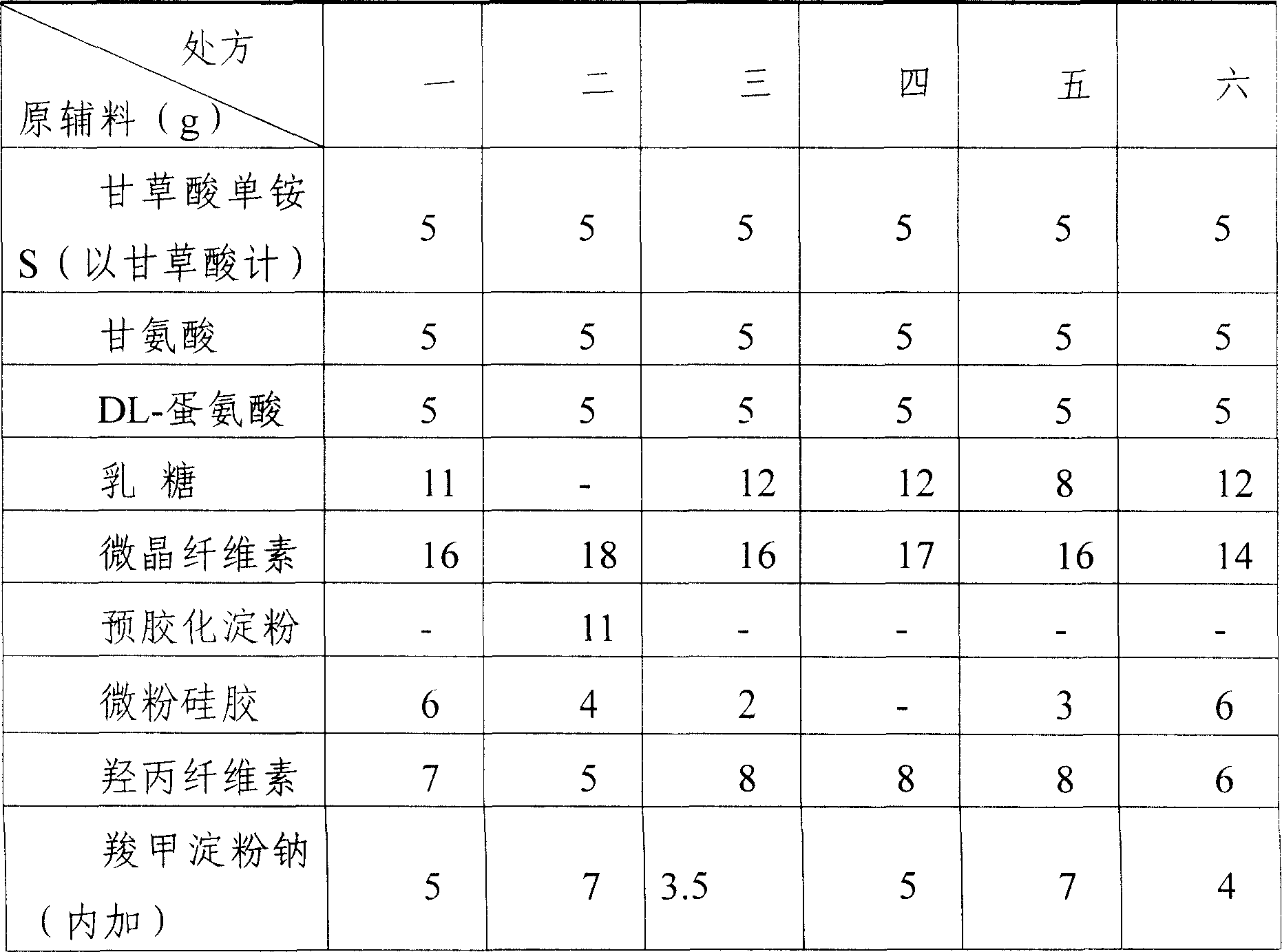
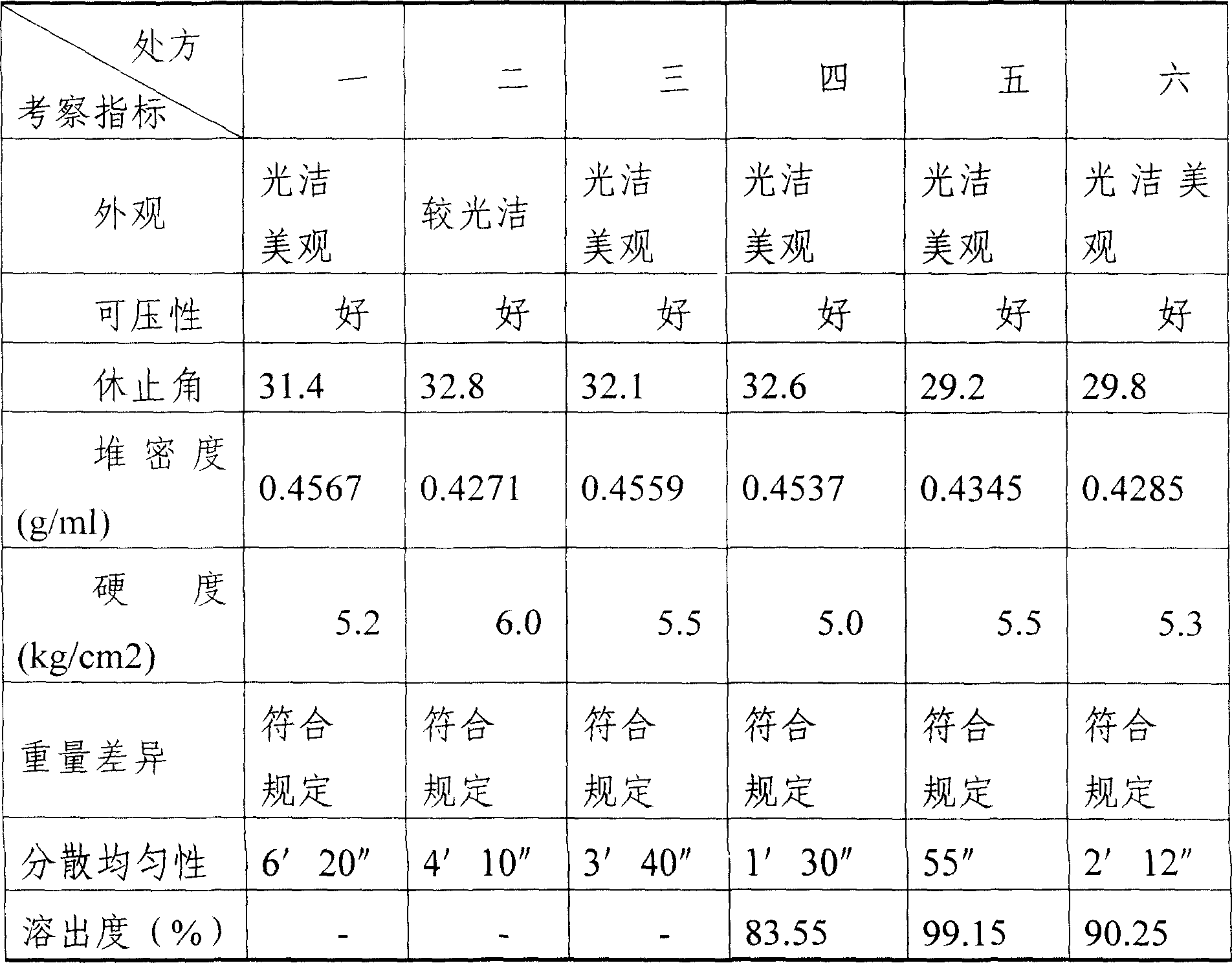
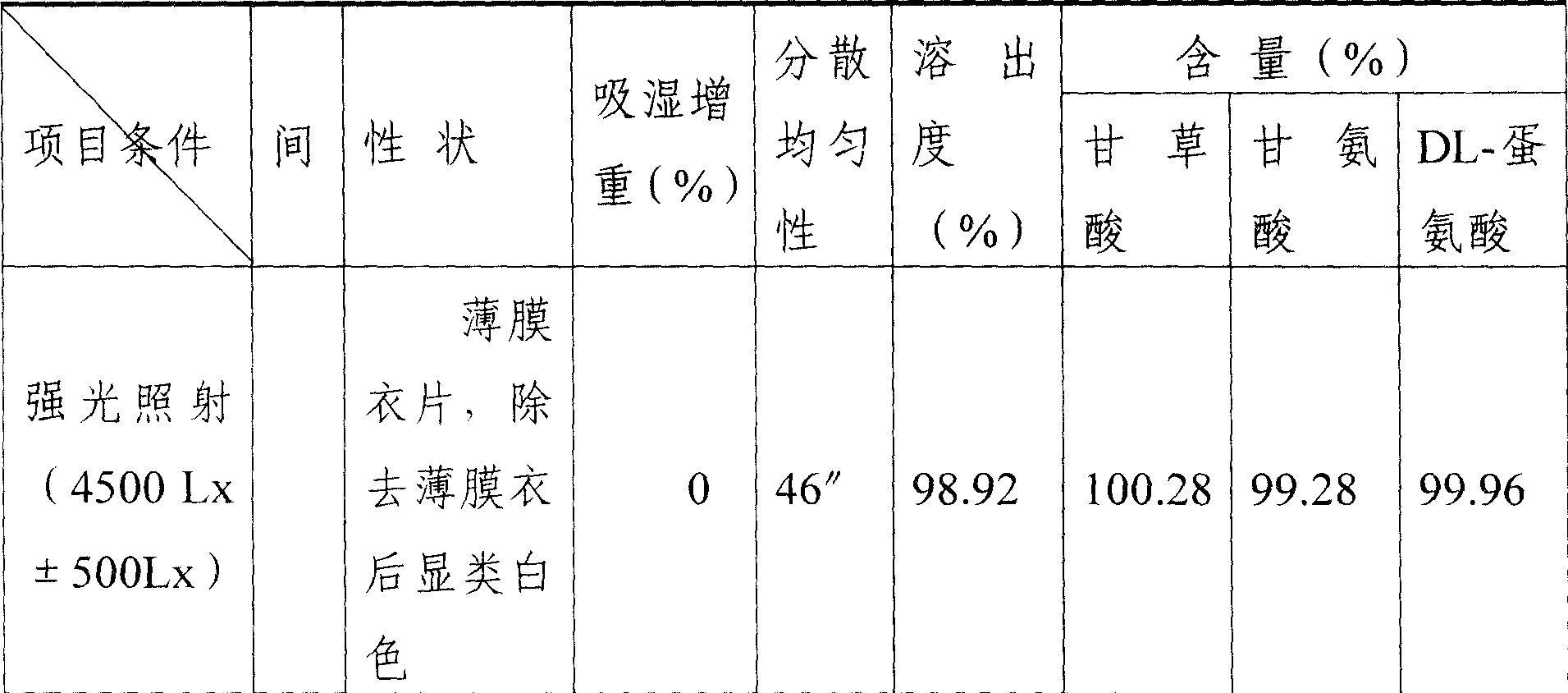
Compound ammonium glycyrrhizinato S dispersed tablet and its preparing process
InactiveCN1919185AImprove complianceSimple production processOrganic active ingredientsDigestive systemGlycineDL-methionine
The invention discloses a compound ammonium glycyrrhizinate S dispersible tablet, wherein the dispersible tablet is prepared from the following raw materials (by weight portion): monoammonium glycyrrhizinate S (calculated by glycyrrhizinic acid) 5-30 parts, glycine 5-30 parts, DL-methionine 5-30 parts, bulking agent 55-220 parts, crumbling agent 32-160 parts, lubricating agent 0.2-5 parts. The dispersible tablet has the advantages of shorter disintegration time, better dispersion state, faster medicament dissolving, quicker absorption, and higher biological availability, thus is especially suitable for the elder people and patients suffering from swallowing difficulty.
Owner:黄本东
Raspberry health product and preparation technique thereof
InactiveCN101558876AGuaranteed Bioavailability and Health BenefitsGood for oral administration and long-term storageFood shapingFood preparationProduction cycleOral medication
The invention relates to a raspberry health product and a preparation technique thereof. The raspberry health product comprises two parts of raw materials and auxiliary materials; wherein the raw materials are raspberry and American ginseng with the proportion of weight portions of usage thereof of 100:8 to 16; the auxiliary materials are a filling and shaping agent, a wetting agent and a lubricating agent. As the new formula and extracting preparation technique are adopted, the invention furthest remains all effective active components in the raw materials, guarantees bioavailability and health effect, is beneficial to oral administration and long-term storage, has simple preparation technique, short production cycle and low production cost and is an ideal and novel health product with combination of raspberry and American ginseng at present.
Owner:JIANGXI FUSHENGYUAN BIOTECH CO LTD
8-amino acid/11-vitamin containing micro granule capsule and preparation method thereof
ActiveCN101773512BImprove bioavailabilityUnique craftPeptide/protein ingredientsHydroxy compound active ingredientsDiseaseLiver and kidney
The invention discloses an 8-amino acid / 11-vitamin containing micro granule capsule and a preparation method thereof, wherein the micro granule capsule contains 7 ball micro granules consisting of the following raw materials in part by weight: 2.66-9.9 parts of thiamine mononitrate micro granule, 16.35-20.5 parts of vitamin micro granule a, 35.0-44.1 parts of amino acid micro granule a, 11.4-14.4parts of vitamin micro granule b, 6.65-10.5 parts of amino acid micro granule b, 12.73-16.0 parts of vitamin C micro granule and 5.7-9.36 parts of vitamin AD micro bead. The method has the advantages of scientific and reasonable proportion, good stability of products, no toxicity and side effect and excellent biological effect, and can be used for the preparation of medicines treating chronic liver and kidney diseases and of food alleviating and eliminating physical fatigue caused by sports training.
Owner:SHENZHEN WANHE PHARMA
Preparation method of olmesartan medoxomil tablets
InactiveCN107773544ARapid dissolutionPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsAdjuvantMedicine
The invention discloses a method for preparing olmesartan medoxomil tablets, comprising: (1) mixing olmesartan medoxomil with water-soluble materials, and then pulverizing them; (2) pulverizing the olmesartan medoxomil tablets obtained in step (1) The mixture is mixed with one or more auxiliary agents, and then directly compressed to obtain olmesartan medoxomil tablets. The invention has the advantages of simple operation, short process steps, strong controllability, good reproducibility, convenient industrial production and cost saving.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Medicament for supplying DE calcium and preparation method thereof
InactiveCN101757000ASmall toxicityMedication safetyMetabolism disorderPill deliveryIntestinal structureSide effect
The invention provides a medicament for supplying DE calcium and a preparation method thereof. The active ingredient of the medicament comprises the slow release grains made up of vitamin D and the double-layer tablet formed by pressing the quick release grains made up of vitamin E and calcium acetate. The preparation method comprises the following steps of: respectively preparing the slow release grains and the quick release grains, mixing with proper superfine silica powder, stirring, and then filling in two hoppers of a double-layer tablet press so as to press the tablet. The medicament prepared by using the method ensures that the vitamin D is not destroyed under the acid condition and the direct contact between the vitamin E and oxygen is prevented. After patient takes the medicine, the active ingredients of vitamin E and calcium acetate can be quickly disintegrated and absorbed in stomach and the vitamin D can be disintegrated and absorbed in intestines. The medicament is characterized by pesticide effect, high bioavailability and small side effect and is specially fit for children, pregnant woman, adult and old people to take for a long time.
Owner:广西强寿药业集团有限公司
Preparation method for producing adenosine-containing lucid ganoderma extract piece by liquid fermentation
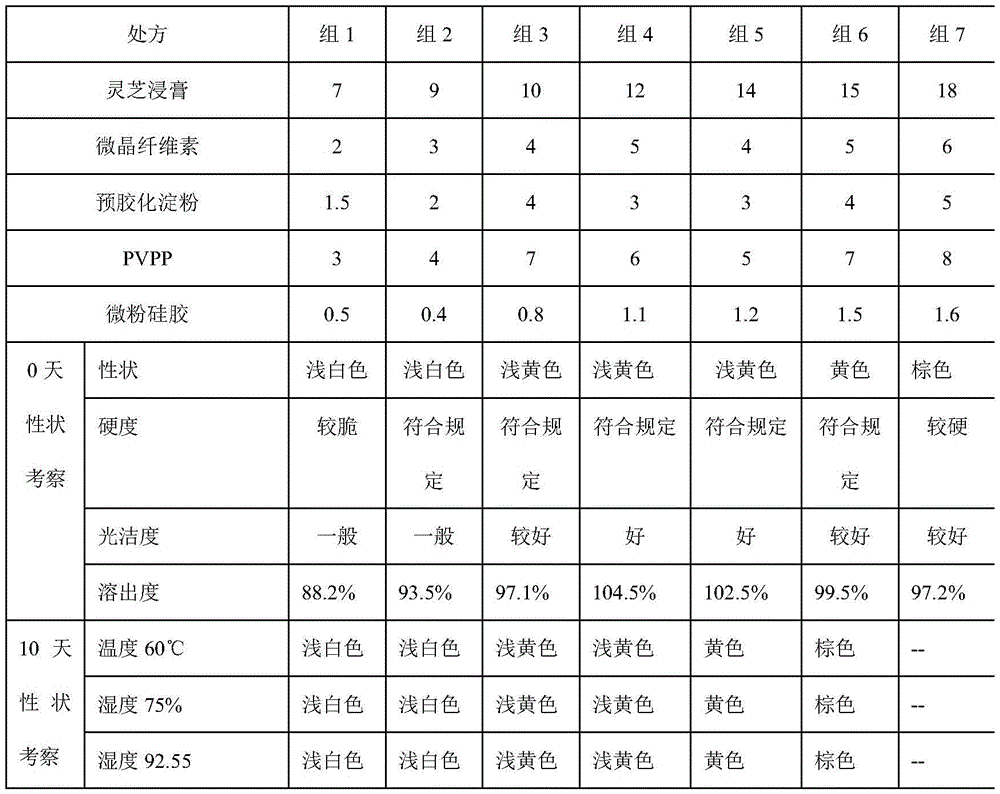
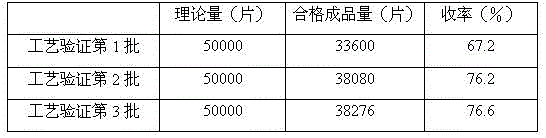
ActiveCN103830283AImprove stabilityHigh content of active ingredientsNervous disorderRespiratory disorderBiotechnologyAdenosine
The invention relates to a preparation method for producing an adenosine-containing lucid ganoderma extract piece by liquid fermentation. The preparation method comprises the following steps: filtering a ganoderma lucidum bacterial liquid fermenting matter and concentrating the filtrate to a thick paste; respectively carrying out microwave-assisted extraction by ethanol on residues twice, combining extracting liquids after extraction twice, recovering ethanol and concentrating to the thick paste, combining with the fermenting liquid and the thick paste, uniformly stirring, performing decompression-concentrating on an extract, and spraying and drying to obtain lucid ganoderma powder; and then adding corresponding auxiliaries to prepare troches. The lucid ganoderma extract piece prepared by adopting the process is good in curative effect, stable in preparation and fewer in side effect, and the content of adenosine in each lucid ganoderma extract piece is not lower than 0.28mg.
Owner:JIANGXI BAISHEN PHARMA
Preparation method of high-uniformity vitamin AD pellets and vitamin AD pellets
ActiveCN109432070ATo promote metabolismImprove bioavailabilityVitamin food ingredientsHydroxy compound active ingredientsVitamina D2Emulsion
The invention relates to a preparation method of high-uniformity vitamin AD pellets and the vitamin AD pellets. Specifically, the vitamin AD pellets involved in one aspect of the invention comprise vitamin A and vitamin D2, and each gram of the pellets comprises 2-200000 I. U. Vitamin A and 0.2-20000 I. U. Vitamin D2.The preparation method of the vitamin AD pellets comprises the following steps of: dissolving an oily material to obtain oily liquid, dissolving an aqueous material to obtain aqueous liquid, emulsifying the mixture in a vacuum emulsifier to obtain emulsion, dropping the emulsion into curing oil through a dropping machine, carrying out cooling and solidification to form pellets, and filtering and drying the pellets to obtain the vitamin AD pellets. The vitamin AD pellets have excellent uniformity in the aspects of three-dimensional size as well as weight and active ingredient content.
Owner:SHENZHEN WANHE PHARMA
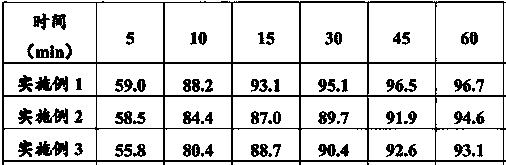
Method for determining in-vitro dissolution of felodipine sustained-release tablets
ActiveCN112526055AOvercome cohesionEnsure physiological temperatureComponent separationDissolutionPharmaceutical Substances
The invention belongs to the technical field of pharmaceutical analysis, and discloses a method for determining in-vitro dissolution of felodipine sustained-release tablets, wherein the method comprises the steps: dissolving out felodipine sustained-release tablets by using a two-chamber model experiment device, dissolving out a pH 6.8 phosphate buffer solution of which the medium is 0.3% lauryl sodium sulfate, and putting the felodipine sustained-release tablets into a rotating basket at the rotating basket rotating speed of 100 rpm; filtering the dissolution solution by a filter membrane, continuously extracting the dissolution solution, and measuring the concentration of the felodipine in the dissolution solution at the extraction speed of the dissolution solution and the supplementingspeed of a dissolution medium of 5-12 mL / min until the dissolution of the felodipine sustained-release tablets is completed; and determining the average dissolution concentration of the felodipine inthe dissolution solution at different time points by adopting a high performance liquid chromatography. According to the in-vitro dissolution method, the in-vivo absorption process is simulated, so that the accuracy of the in-vitro dissolution experiment is guaranteed, the consistency of a dissolution curve measured by the in-vitro dissolution experiment and the in-vivo absorption process is realized, and a guarantee is provided for realizing good prediction of the bioequivalence of a human body.
Owner:HUNAN HUIZE BIO PHARMA CO LTD
A kind of olaparib solid dispersion preparation and preparation method thereof
ActiveCN104434809BWide variety of sourcesEase of industrial productionPowder deliveryOrganic active ingredientsProcess engineeringBioavailability
The invention relates to the field of a pharmaceutical preparation, and in particular discloses an olaparib solid dispersion preparation and a preparation method thereof. The olaparib solid dispersion preparation consists of olaparib, povidone, a special lubricating agent, a special disintegrating agent and a thinner. According to the olaparib solid dispersion preparation disclosed by the invention, povidone, replacing the existing copovidone, is used as a matrix polymer, and appropriate auxiliary materials are added, so that the povidone, as the matrix polymer of the olaparib solid dispersion preparation, is wide in source, low in cost and clear in quality standard in accordance with Chinese Pharmacopoeia; meanwhile, the dissolution effect, bioavailability and stability of the preparation are guaranteed; and the industrial production of the olaparib solid dispersion preparation is facilitated.
Owner:BEIJING COLLAB PHARMA
Azithromycin medicine composition and preparation method thereof
ActiveCN110755404AAdjust the dissolution rateDoes not change the degree of dissolutionAntibacterial agentsOrganic active ingredientsAzithromycinEfficacy
The invention provides an azithromycin medicine composition and a preparation method thereof. The azithromycin medicine composition comprises medicine granules containing azithromycin, an isolation layer, a taste masking layer and a taste modifying layer, wherein the isolation layer, the taste masking layer and the taste modifying layer sequentially coat the medicine granules in a form of coating.The isolation layer, the taste masking layer and the taste modifying layer of the azithromycin medicine composition are prepared into coats to coat sequentially coat the medicine granules in the formof coating, so that a medicine component release curve is more gentle, and the occurrence of adverse reactions of gastrointestinal tracts can be reduced effectively on the premise that the efficacy and bioavailability are guaranteed. Meanwhile, the azithromycin medicine composition reduces the dosage of a taste modifying agent, is safer to take by children and keeps a good taste.
Owner:长春雷允上药业有限公司
Stable cefeclor dispersible tablet and preparation method thereof
ActiveCN101912373BLong validity periodImprove bioavailabilityAntibacterial agentsOrganic active ingredientsCross-linkHypromellose
The invention belongs to the technical field of medicament preparations, and particularly relates to a cefeclor dispersible tablet which is characterized by being prepared from the following components in parts by weight: 25g of cefeclor, 11.5g of microcrystalline cellulose, 5-7g of hydroxypropyl cellulose, a proper amount of 2-percent hydroxypropyl methylcellulose 40-percent ethanol solution, 5gof pregelatinized starch, 2.8-4g of cross-linked polyvidone, 1.2g of silicon dioxide, 1-2g of magnesium laurylsulfate and 0.12g of essence. Because the magnesium laurylsulfate is used in the dispersible tablet, the preparation stability is markedly improved, the content is basically not changed after long-time placement, and the service time of the medicament is prolonged.
Owner:BEIJING JINGFENG PHARMA GRP
Clarithromycin enteric-coated preparation and preparation method thereof
ActiveCN103239406BWill not degradeGood dispersionAntibacterial agentsOrganic active ingredientsCurative effectClarithromycin
Owner:HAINAN PULIN PHARMA +1
Vitamin AD pellet and compound amino acid capsule composition containing same
ActiveCN106902100AImprove bioavailabilityUnique craftHydroxy compound active ingredientsMetabolism disorderAmino acidVitamina D2
The invention relates to a vitamin AD pellet and a compound amino acid capsule composition containing the same. Specifically, the invention relates to the vitamin AD pellet, and each g of the vitamin AD pellet contains 20,000 to 200, 000 IU of vitamin A and 2, 000 to 20,000 IU of vitamin D2. The invention also relates to a preparation method for the vitamin AD pellet and a compound amino acid-vitamin pellet capsule containing the vitamin AD pellet. The vitamin AD pellet and the capsule prepared from the same have characteristics superior to conventional products.
Owner:SHENZHEN WANHE PHARMA
Tolvaptan oral preparation and preparation method thereof
ActiveCN113171344AGuaranteed bioavailabilityReduce precipitationPowder deliveryPharmaceutical non-active ingredientsOrganosolvExcipient
The invention relates to a tolvaptan oral preparation and a preparation method thereof. The preparation method comprises the following steps: firstly, dispersing a tolvaptan bulk drug into a carrier-hydroxypropyl cellulose and an organic solvent; dispersing the tolvaptan bulk drug in a carrier-hydroxypropyl methylcellulose phthalate and an organic solvent, respectively carrying out spray drying by adopting an explosion-proof closed nitrogen circulation spray dryer, and strictly controlling process parameters in the spray drying process, and drying under reduced pressure to prepare two solid dispersions of tolvaptan: a water-soluble solid dispersion and an enteric solid dispersion, wherein the two solid dispersions are then combined with excipients to prepare tolvaptan oral preparations, the quick-release solid dispersion is dissolved in the stomach, the enteric-soluble solid dispersion is dissolved in the intestinal tract, so that the condition of precipitation caused by overhigh local concentration and oversaturation is reduced, and the bioavailability of the medicine can be better ensured.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
Azithromycin sustained-release microsphere and dry suspension, preparation method and application thereof
ActiveCN102058540BReduce dosageExtrude evenlyAntibacterial agentsOrganic active ingredientsAzithromycinOrganic solvent
The invention discloses an azithromycin sustained-release microsphere which comprises 30-70% of azithromycin, 5-25% of wax ester sustained-release framework material, 5-45% of other water-insoluble framework material except the wax ester sustained-release framework material and 0.1-25% of binder based on the total weight of the azithromycin sustained-release microsphere, wherein the binder is aqueous dispersion coating fluid, and the weight percentage of the binder is the weight percentage of solid substances except the solvent in the binder. The invention also discloses a preparation method and application of the azithromycin sustained-release microsphere, a suspension containing the azithromycin sustained-release microsphere and application of the suspension. According to the invention,the defect that the spraying-congelation or a large amount of organic solvent or coating is required in the prior art is solved, and the invention is particularly suitable for the preparation with anenvironmentally friendly and nontoxic extrusion-rounding method with simple steps.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
A kind of preparation method of Ganoderma lucidum extract sheet
ActiveCN103816191BImprove stabilityHigh content of active ingredientsNervous disorderDigestive systemSide effectCurative effect
The invention relates to a preparation method of a ganoderma lucidum extract tablet. The preparation method comprises the following steps: taking a ganoderma lucidum liquid fermented extract, filtering, and concentrating the filtrate to obtain a stiff paste; carrying out microwave-assisted extraction on residues with ethanol for two times, merging extracts obtained by the two times of microwave-assisted extraction, recovering ethanol and concentrating to obtain a stiff paste, merging the stiff paste with the above fermentation liquid paste, uniformly stirring, carrying out pressure reduction and concentrating to obtain a clear paste; and adding corresponding auxiliary materials to prepare the tablet. The ganoderma lucidum extract tablet prepared by the technology has advantages of good curative effect, stable preparation and little side effect.
Owner:JIANGXI BAISHEN PHARMA
A kind of rupatadine fumarate tablet
ActiveCN103120650BImprove discoloration, etc.Promote dissolutionOrganic active ingredientsPill deliveryLactoseColor changes
The invention provides a rupatadine fumarate tablet. Each 1000 tablets comprise 12.8g of rupatadine fumarate, 30-120g of lactose, 3-15g of pregelatinized starch, 3-15g pf hydroxypropylcellulose, 0.03-0.30g of ferric oxide and 0.3-12g of wetting agent. According to the embodiment of the invention, looseness and color change and the like can be improved, and indissolvable main medicine is dissolved quickly, so that the in vivo bioavailability is ensured, and furthermore, good effect in clinical use is obtained.
Owner:海思科制药(眉山)有限公司
Pharmaceutical composition of lenvatinib, and applications thereof
InactiveCN111053772AGuaranteed bioavailabilityPromote dissolutionInorganic non-active ingredientsAntineoplastic agentsSodium bicarbonateLenvatinib
The invention provides a pharmaceutical composition, which contains lenvatinib or a pharmaceutically acceptable salt thereof, sodium carbonate and sodium bicarbonate. According to the invention, the pharmaceutical composition solves the problem of difficulty in dissolution of lenvatinib, has excellent dissolution behavior, improves the bioavailability of the drug, has excellent stability, and canensure clinical medication safety.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Compound amino acid capsule
ActiveCN105663107AImprove bioavailabilityPromote biosynthesisHydroxy compound active ingredientsMetabolism disorderDiseaseSide effect
The invention relates to a compound amino acid capsule. The compound amino acid capsule comprises a capsule shell and powdery or granular filler wrapped in the capsule shell, wherein the capsule shell is a hollow gelatin capsule prepared from gelatin as a main component; the filler comprises active substances and pharmaceutic adjuvants. The compound amino acid capsule adopts a scientific and reasonable ratio, has good product stability, is free of toxic and side effects, has high safety and good biological effect, and can be applied to preparation of drugs for treating chronic liver and kidney diseases and food for relieving and eliminating physical fatigue caused by exercise training. The capsule has the characteristics superior to those in the prior art.
Owner:李兴惠
Water-soluble fertilizer and preparation method thereof
PendingCN112028703AGuaranteed BioavailabilityGood slow release propertiesAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersChemistrySoil science
The water-soluble fertilizer is prepared from the following raw materials in parts by weight: 17 to 50 parts of ammonium polyphosphate, 10 to 60 parts of organic potassium salt, 10 to 40 parts of urea, 1 to 5 parts of sodium octaborate tetrahydrate, 1 to 5 parts of EDTAzinc, 10 to 30 parts of glacial acetic acid, 1 to 5 parts of xanthan gum and 1 to 3 parts of tea polyphenol. According to the water-soluble fertilizer provided by the invention, all the raw materials are matched for use according to a certain proportion, so that the proportion of nitrogen, phosphorus and potassium and trace elements can be optimized, the microbial environment of soil can be improved, the utilization rate of applied nitrogen, phosphorus and potassium by crops is increased, and the yield-increasing effect of the crops is more remarkable. The invention also provides a preparation method of the water-soluble fertilizer.
Owner:普罗丰禾(武汉)科技有限公司
Method for preparing vitamin ad pellets and compound amino acid capsule composition
ActiveCN106821998BImprove bioavailabilityUnique craftHydroxy compound active ingredientsMetabolism disorderVitamin A AlcoholVitamin a1
The invention relates to a method for preparing a vitamin AD pellet, and a compound amino acid capsule composition thereof. Concretely, on one hand, the invention relates to the vitamin AD pellet, which comprises vitamin A and vitamin D2; every 1g of pellet contains 2 to 200,000 I.U. of vitamin A and 0.2 to 20,000 I.U. of vitamin D2. The invention also relates to the preparation method of the vitamin AD pellet, which comprises a capsule of a compound amino acid vitamin pellet containing the vitamin AD pellet. The vitamin AD pellet and the compound amino acid vitamin capsule prepared by using the vitamin AD pellet have the characteristics of superior to existing products.
Owner:SHENZHEN WANHE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com