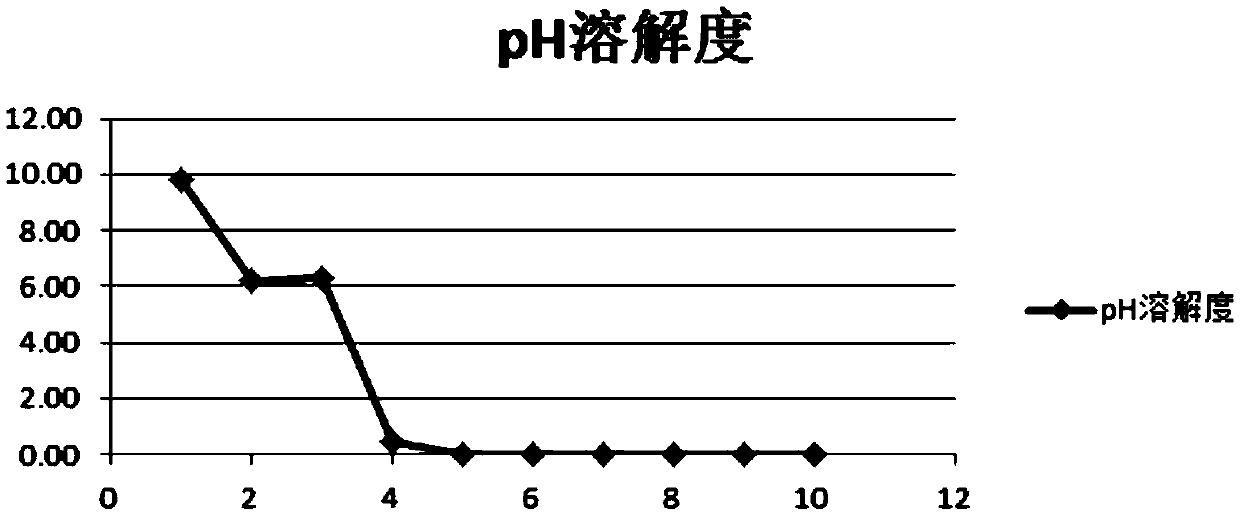
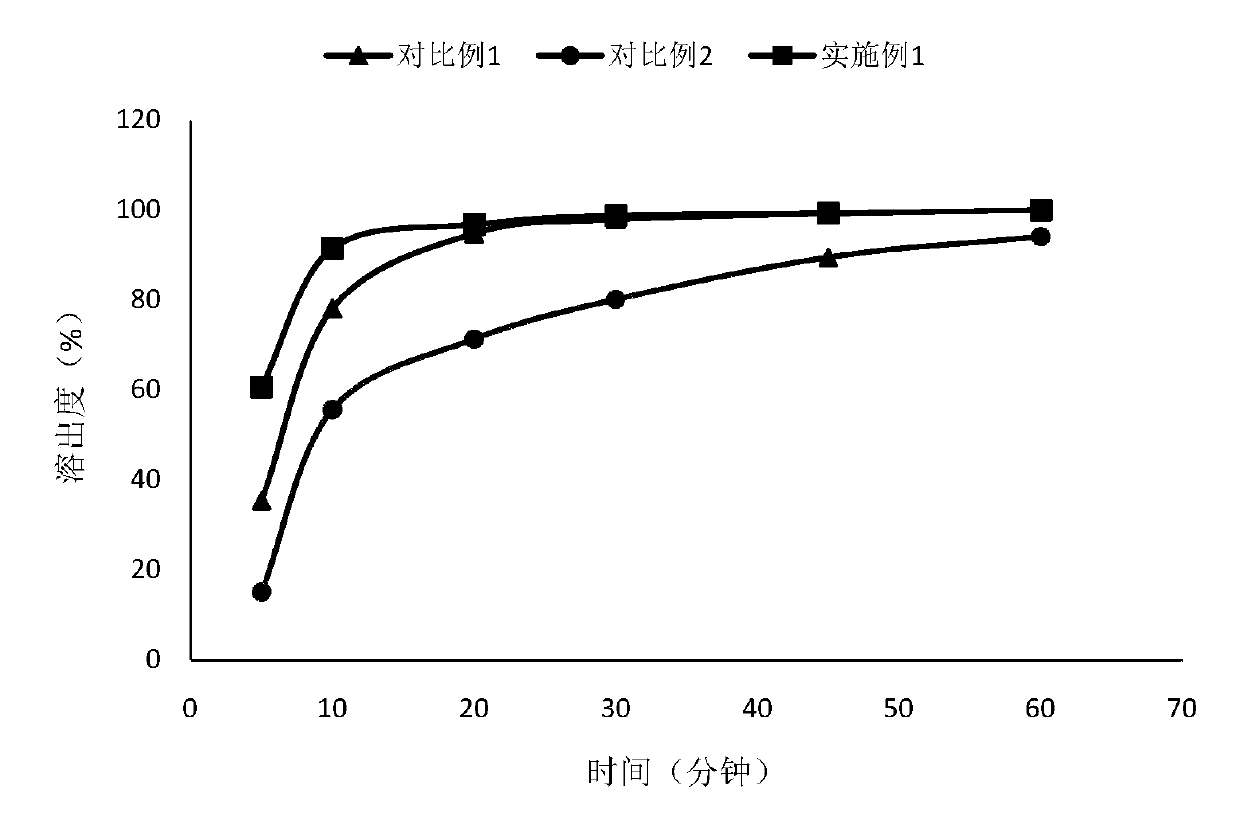
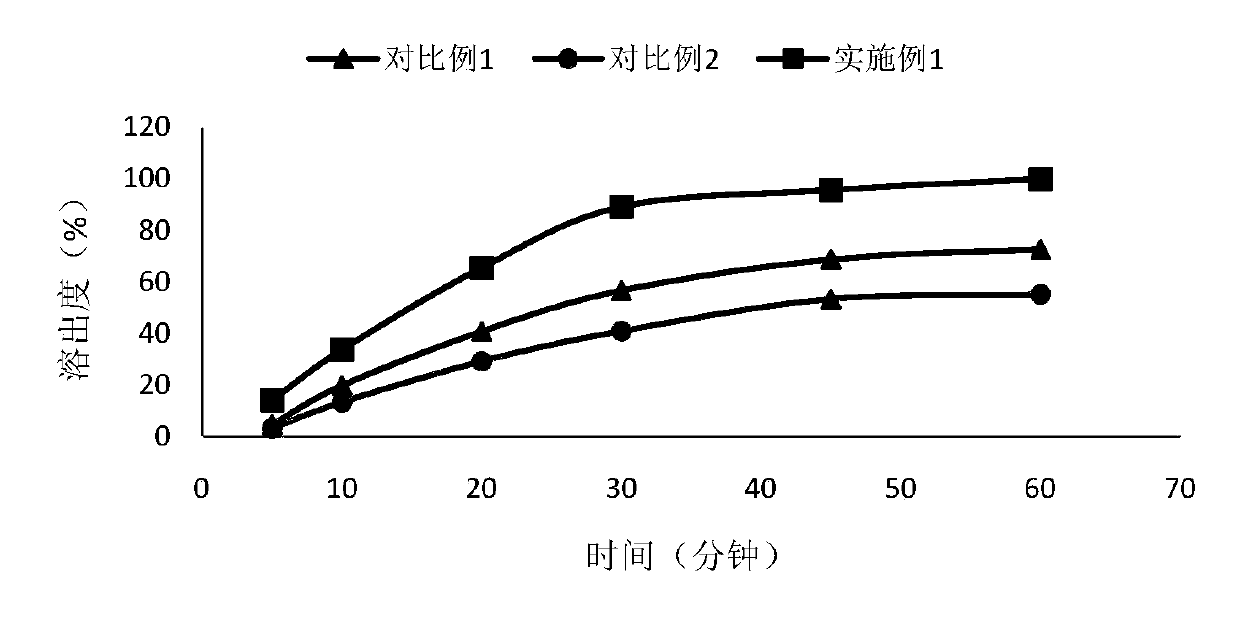
Pharmaceutical composition of lenvatinib, and applications thereof
A technology of lenvatinib and a composition, applied in the field of pharmaceutical preparations, can solve the problems of insufficient calcium hydrogen phosphate to improve product stability, easy gelation of raw materials, growth of degraded impurities, etc., to ensure bioavailability and rapid dissolution , the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0096] Preparation prescription (1000 capsules):
[0097]
[0098] The preparation method is as follows:
[0099] Take the prescribed amount of lenvatinib mesylate (D90: 40 μm), sodium bicarbonate (D90: 200 μm), sodium carbonate (D90: 200 μm), lactose, hydroxypropyl cellulose, microcrystalline cellulose KG802, cross-linked polymer Mix evenly in a vinylpyrrolidone mixer, add appropriate amount of ethanol to granulate, dry the granules in an oven below 60°C, control the water content to <3%, add microcrystalline cellulose PH302 and talc powder, mix evenly, and pack into capsules (capsule No. 4).
Embodiment 16
[0101] Preparation prescription (1000 capsules):
[0102]
[0103] The preparation method is as follows:
[0104]Take the prescribed amount of lenvatinib mesylate (D90: 40 μm), sodium bicarbonate (D90: 200 μm), sodium carbonate (D90: 200 μm), starch, povidone k30, and sodium starch glycolate and mix them evenly in a mixer, Add appropriate amount of ethanol to granulate, dry the granules in an oven below 60°C, control the water content to <3%, add microcrystalline cellulose PH302 and magnesium stearate, mix evenly, and pack into capsules (capsule No. 4).
Embodiment 17
[0106] Preparation prescription (1000 capsules):
[0107]
[0108]
[0109] The preparation method is as follows:
[0110] Take the prescribed amount of lenvatinib mesylate (D90: 40 μm), sodium bicarbonate (D90: 200 μm), sodium carbonate (D90: 200 μm), starch, povidone k30, and sodium starch glycolate and mix them evenly in a mixer, Add appropriate amount of ethanol to granulate, dry the granules in an oven below 60°C, control the water content to <3%, add microcrystalline cellulose PH302 and magnesium stearate, mix evenly, and pack into capsules (capsule No. 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com