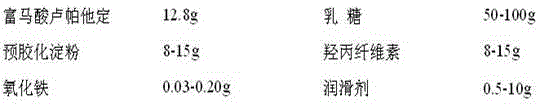A kind of rupatadine fumarate tablet
A technology of rupatadine fumarate and tablets, which is applied in the directions of pill delivery, medical preparations of inactive ingredients, organic active ingredients, etc., to achieve good effects, ensure bioavailability, and improve the effect of loose tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1-tablet of the present invention
[0029] prescription:
[0030]
[0031] Preparation method:
[0032] 1. Pass rupatadine fumarate through a 100-mesh sieve, and pulverize lactose, pregelatinized starch, and hydroxypropyl cellulose through a 80-mesh sieve for later use;
[0033] 2. Take Povidone K 30 , add an appropriate amount of 85% ethanol to dissolve, and make a 10% solution for later use;
[0034] 3. Mix red iron oxide and yellow iron oxide, and mix them with appropriate amount of lactose in equal increments, then add rupatadine fumarate, pregelatinized starch, hydroxypropyl cellulose and remaining lactose, mix evenly, add 10 % povidone K 30 Appropriate amount of 85% ethanol solution is made into soft material, granulated with 20 mesh sieve, dried at 50°C-70°C, moisture is controlled at 1.0%-3.0%, and granulated with 20 mesh sieve;
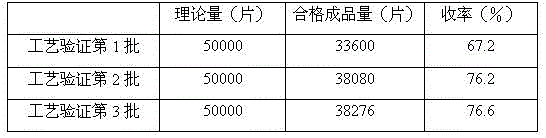
[0035] 4. Add magnesium stearate and talcum powder and mix evenly, measure the particle c...
Embodiment 2
[0039] The preparation of embodiment 2-tablet of the present invention
[0040] prescription:
[0041]
[0042] Preparation method:
[0043] 1. Pass rupatadine fumarate through a 100-mesh sieve, and pulverize lactose, pregelatinized starch, and hydroxypropyl cellulose through a 80-mesh sieve for later use;
[0044] 2. Take Povidone K 30 , add an appropriate amount of 85% ethanol to dissolve, and make a 10% solution for later use;
[0045] 3. Mix red iron oxide and yellow iron oxide, and mix them with appropriate amount of lactose in equal increments, then add rupatadine fumarate, pregelatinized starch, hydroxypropyl cellulose and remaining lactose, mix evenly, add 10 % povidone K 30 Appropriate amount of 85% ethanol solution is made into soft material, granulated with 20 mesh sieve, dried at 50°C-70°C, moisture is controlled at 1.0%-3.0%, and granulated with 20 mesh sieve;
[0046] 4. Add magnesium stearate and talcum powder and mix evenly, measure the particle conten...
Embodiment 3
[0050] Embodiment 3-preparation of tablet of the present invention
[0051] prescription:
[0052]
[0053] Preparation method:
[0054] 1. Pass rupatadine fumarate through a 100-mesh sieve, and pulverize lactose, pregelatinized starch, and hydroxypropyl cellulose through a 80-mesh sieve for later use;
[0055] 2. Take Povidone K 30 , add an appropriate amount of 85% ethanol to dissolve, and make a 10% solution for later use;
[0056] 3. Mix red iron oxide and yellow iron oxide, and mix them with appropriate amount of lactose in equal increments, then add rupatadine fumarate, pregelatinized starch, hydroxypropyl cellulose and remaining lactose, mix evenly, add 10 % Povidone K 30 Appropriate amount of 85% ethanol solution is made into soft material, granulated with 20 mesh sieve, dried at 50°C-70°C, moisture is controlled at 1.0%-3.0%, and granulated with 20 mesh sieve;
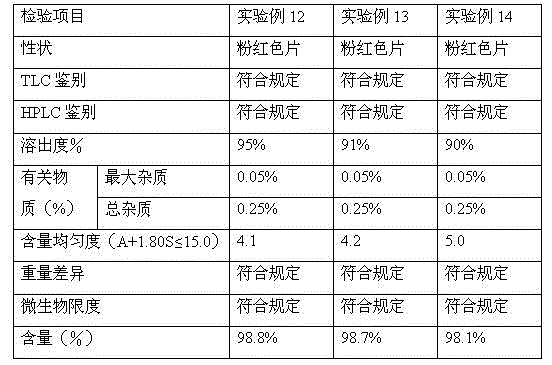
[0057] 4. Add magnesium stearate and stearic acid and mix evenly, measure the particle content, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com