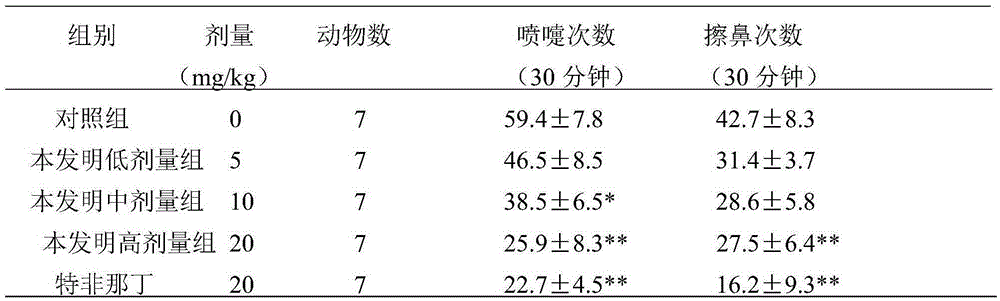
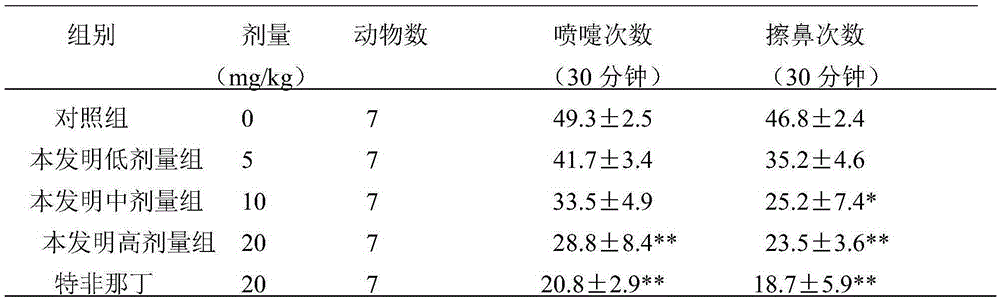
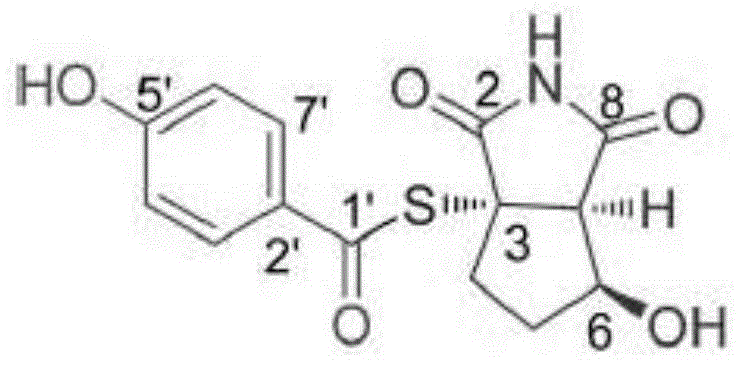
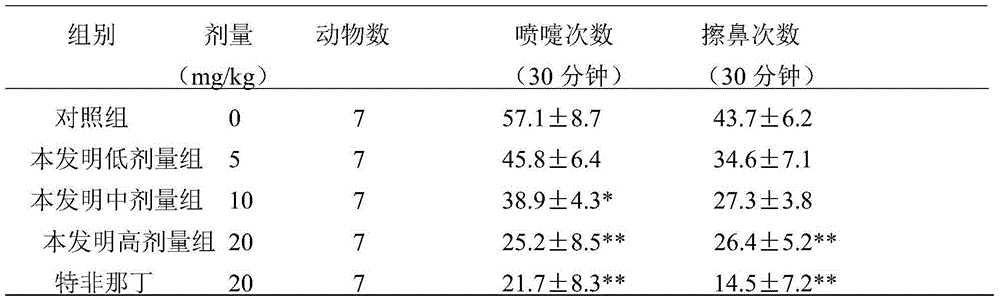
Patents
Literature
70 results about "Terfenadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Terfenadine is an antihistamine formerly used for the treatment of allergic conditions. It was brought to market by Hoechst Marion Roussel (now Sanofi-Aventis) and was marketed under various brand names, including Seldane in the United States, Triludan in the United Kingdom, and Teldane in Australia. It was superseded by fexofenadine in the 1990s due to the risk of a particular type of disruption of the electrical rhythms of the heart (specifically cardiac arrhythmia caused by QT interval prolongation) and has been withdrawn from markets worldwide.
Hydroaminomethylation of olefins
ActiveUS20050215825A1Organic compound preparationCarboxylic acid amides preparationSyngasFexofenadine
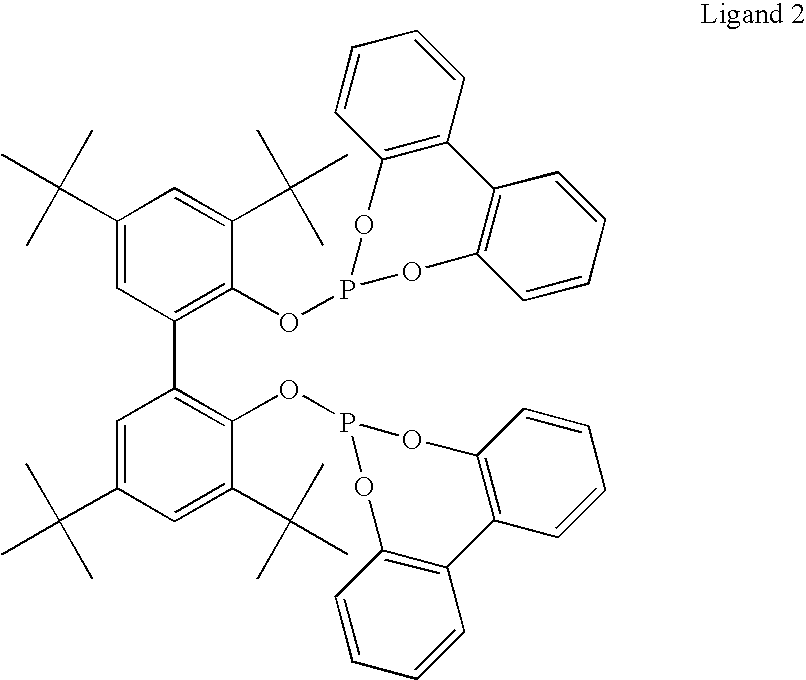
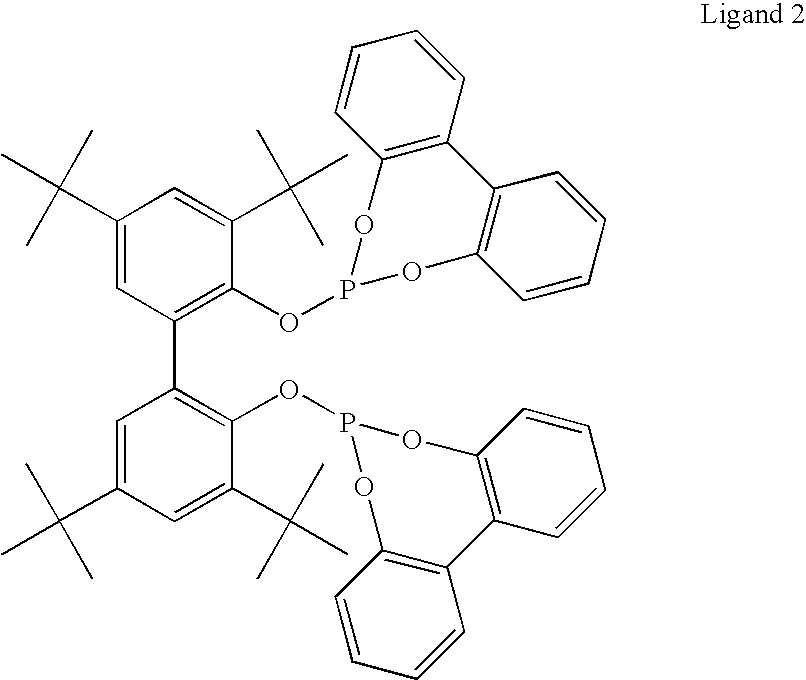
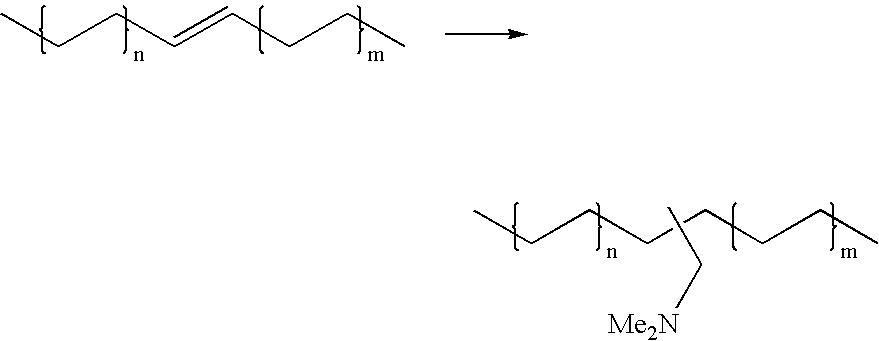
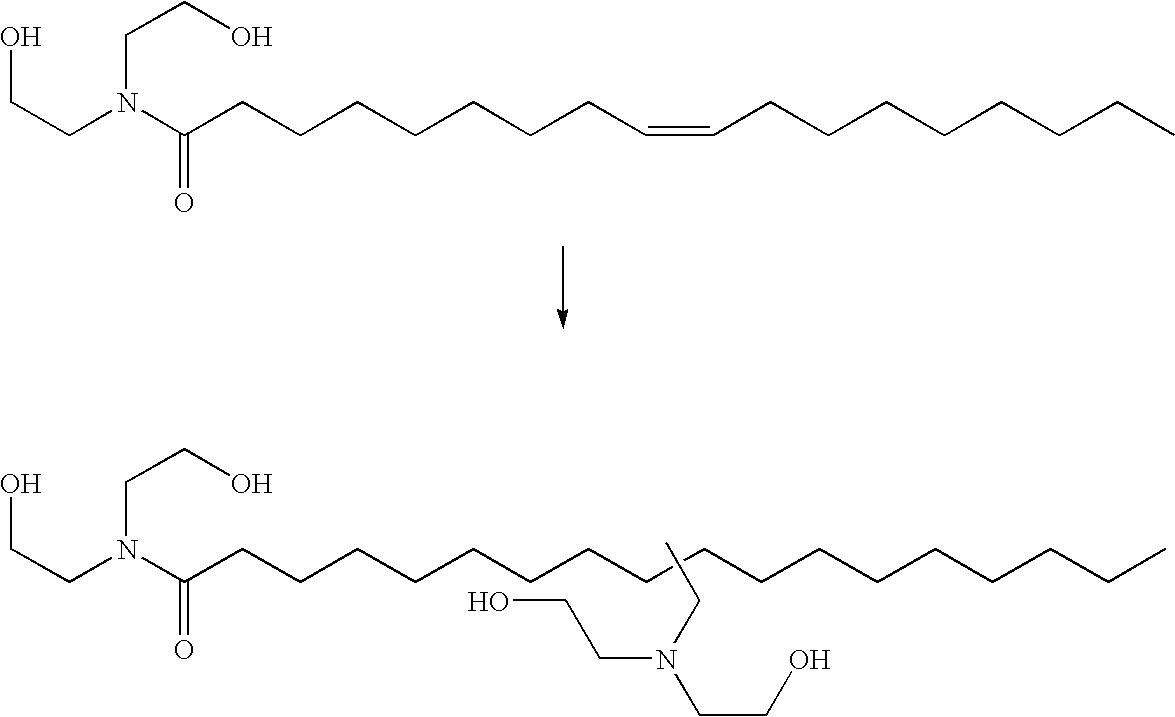
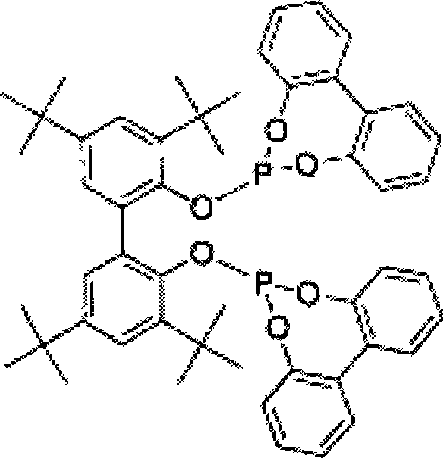
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CORP
Hydroaminomethylation of olefins
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CORP
Hydroaminomethylation of olefins
InactiveCN1918110APreparation by reductive alkylationAmino compound preparation by condensation/addition reactionsSyngasFexofenadine
The present invention relates to a method comprising the step of contacting under hydroaminomethylation conditions, an olefin, an amine, a rhodium-phosphorous ligand, and synthesis gas (syngas). In particular, it has been discovered that, under some circumstances, a neutral rhodium-monodentate phosphite ligand is prescribed. The invention provides a simple way of making, in high yields and regiospecificity, a variety of products, including pharmacologically active products such as ibutilide, terfenadine, and fexofenadine, and derivatives thereof.
Owner:UNION CARBIDE CHEM & PLASTICS TECH CORP
Application of p-hydroxyphenylacetic acid in prevention and/or treatment of cardiovascular diseases
ActiveCN111743885APromote generationIncrease the number of spawnsOrganic active ingredientsCardiovascular disorderVascular endotheliumPhenylacetic acid
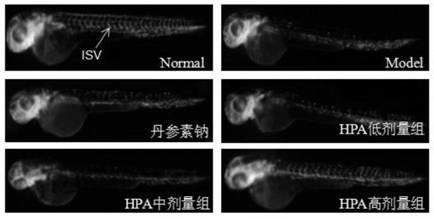
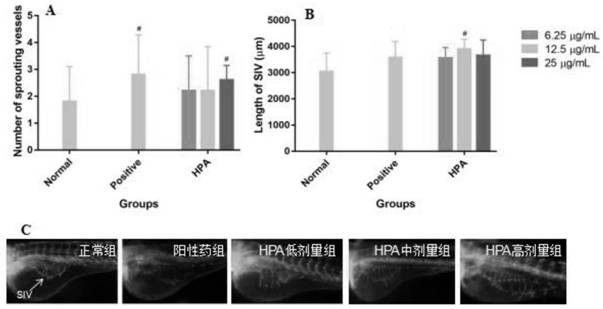
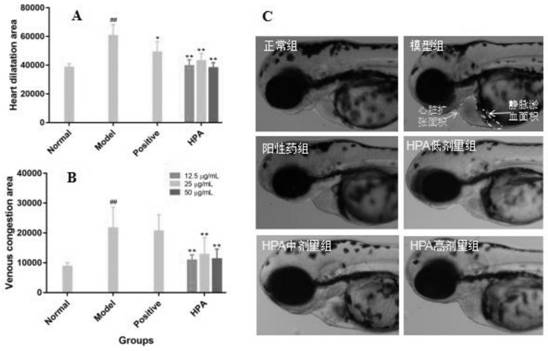
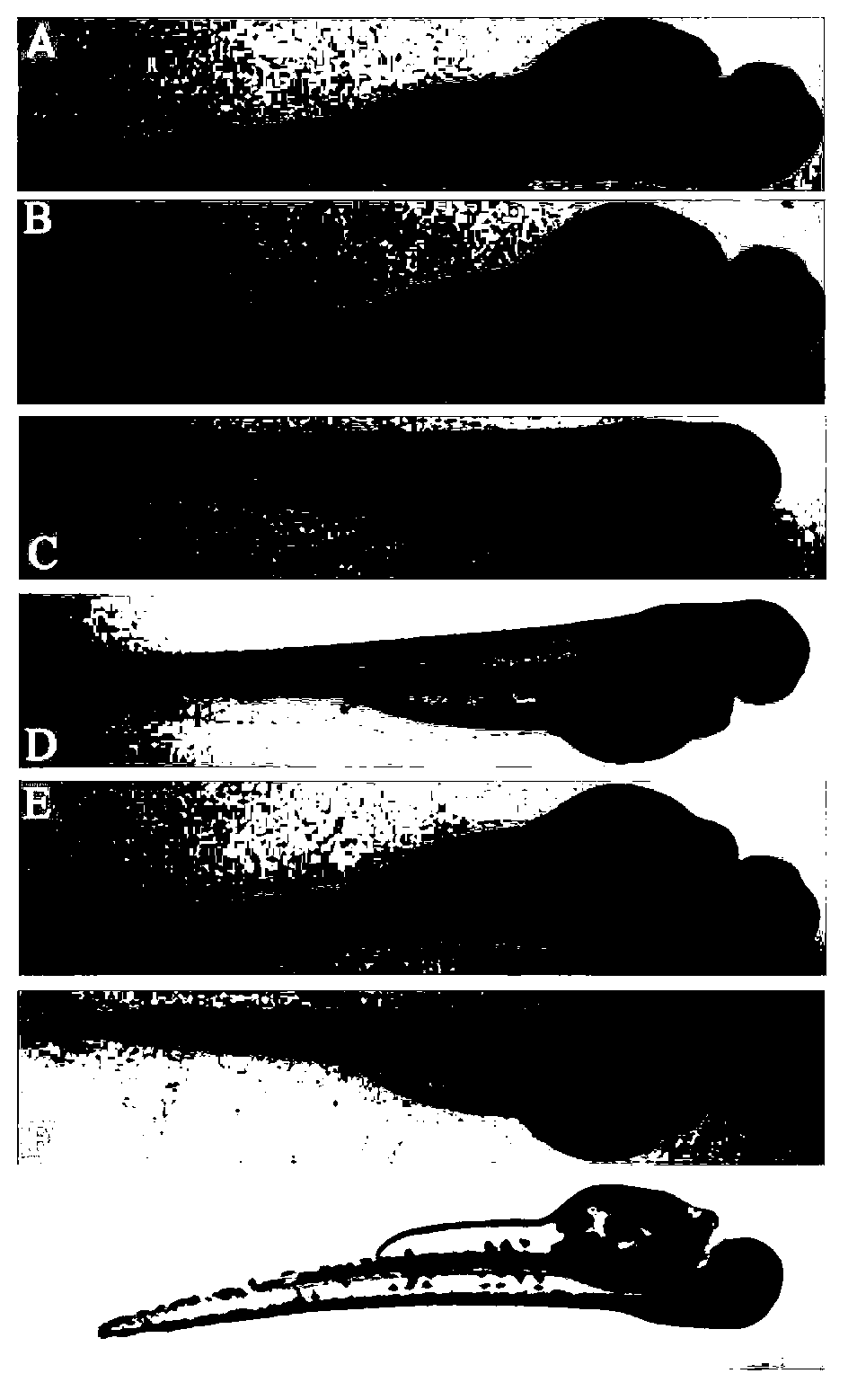
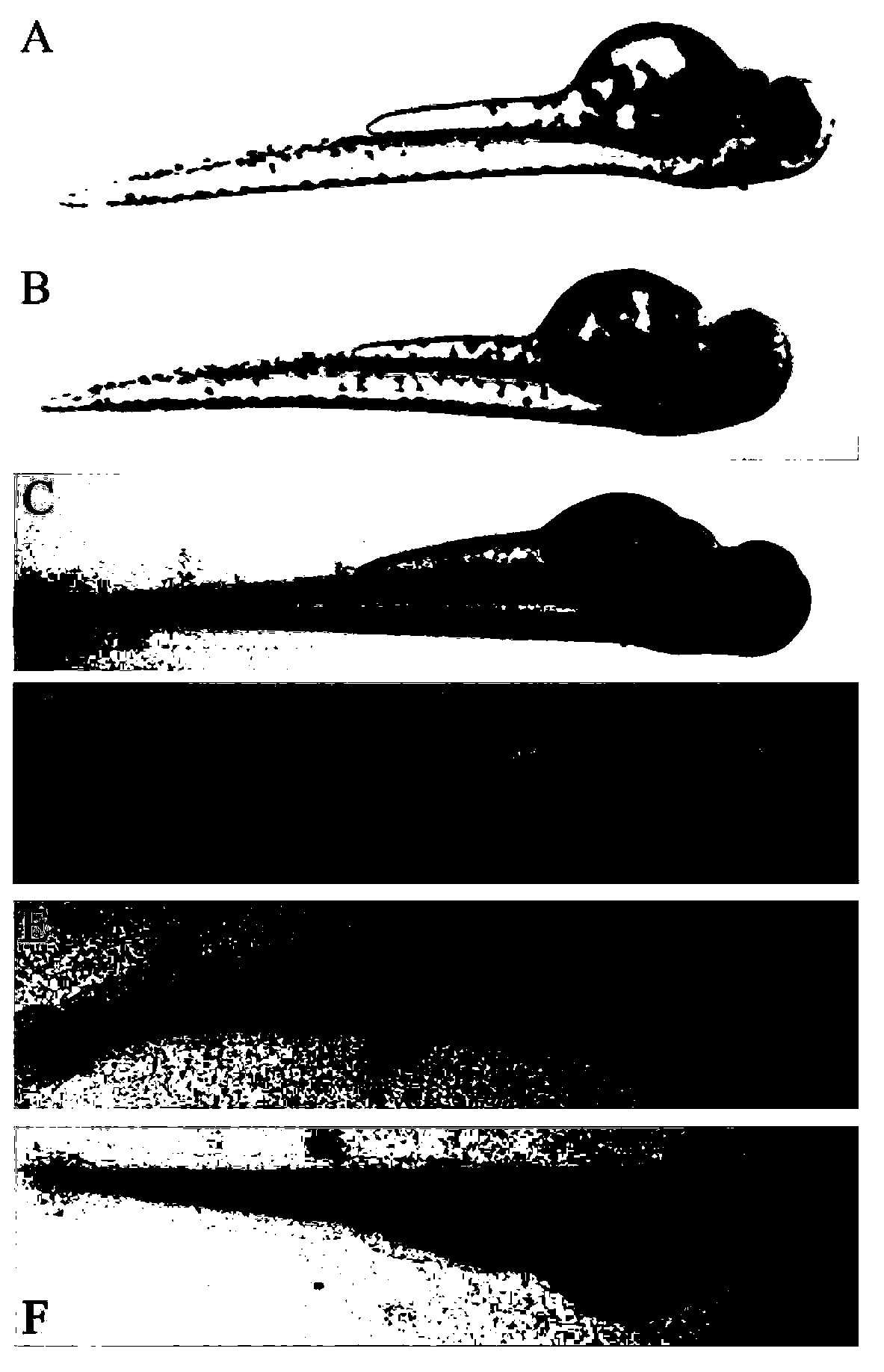
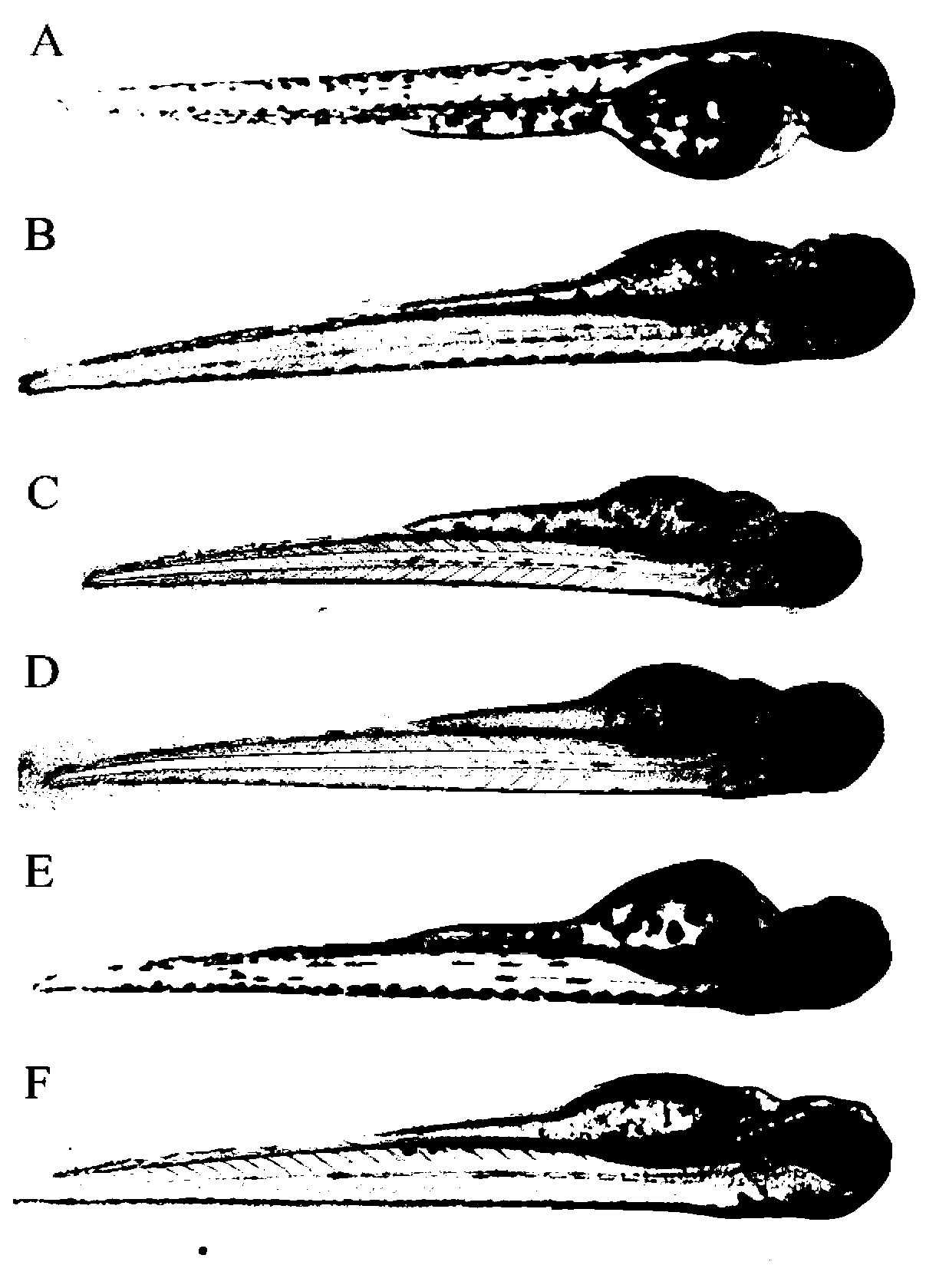
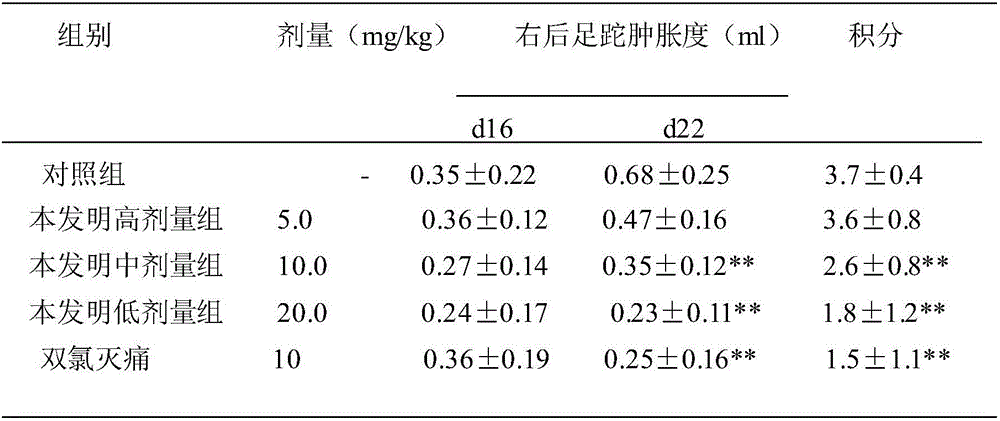
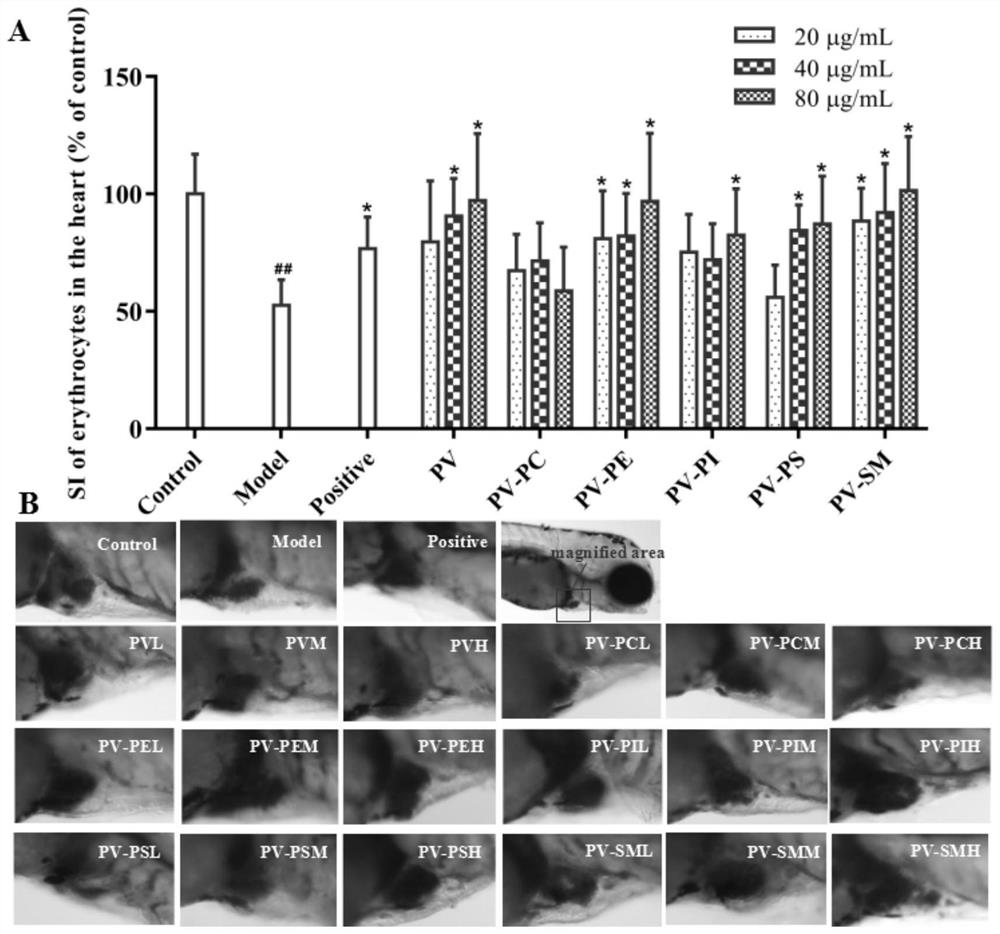
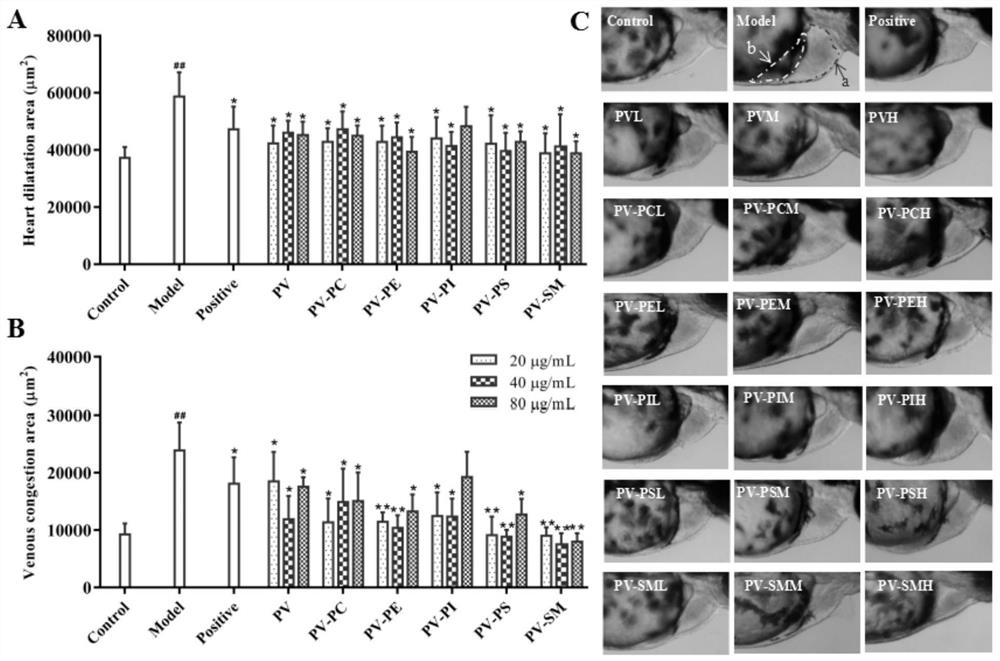
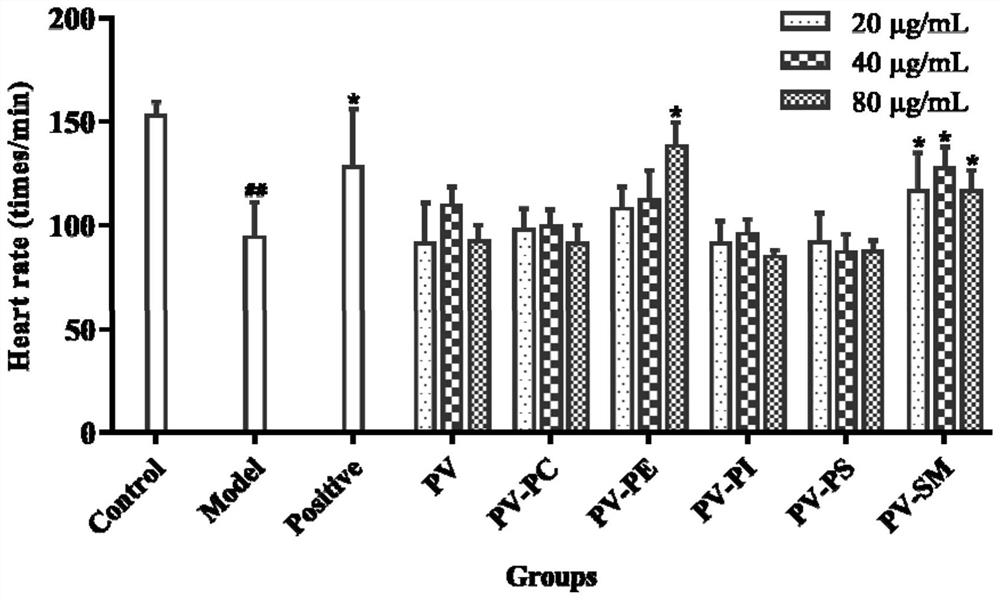
The invention relates to an application of p-hydroxyphenylacetic acid in prevention and / or treatment of cardiovascular diseases. The invention finds for the first time that p-hydroxyphenylacetic acidhas a good treatment effect on vascular and cardiac injuries. For zebra fish interbody vascular (ISV) injury and subintestinal vein (SIV) growth conditions induced by a vascular endothelial cell growth factor receptor inhibitor (PTK787), p-hydroxyphenylacetic acid has a remarkable effect of promoting angiogenesis, and the generation number and the length of blood vessels can be obviously increased. The traditional Chinese medicine composition has a remarkable heart protection effect on heart failure of zebra fish induced by verapamil, arrhythmia induced by terfenadine and p-hydroxyphenylaceticacid, and can obviously reduce cardiac dilatation, relieve venous congestion and promote the heart rate to tend to be normal.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
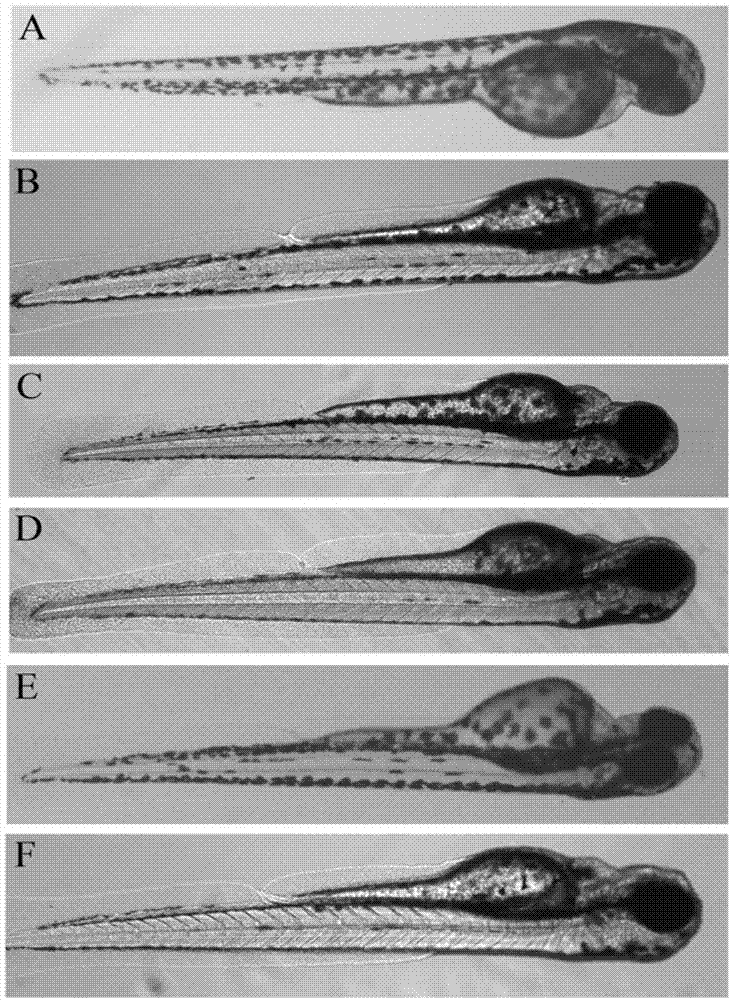
Application of terfenadine in preparation of zebra fish heart function damage model
ActiveCN103230397AEasy to manufactureFast preparationCardiovascular disorderHeterocyclic compound active ingredientsExperimental researchFishery
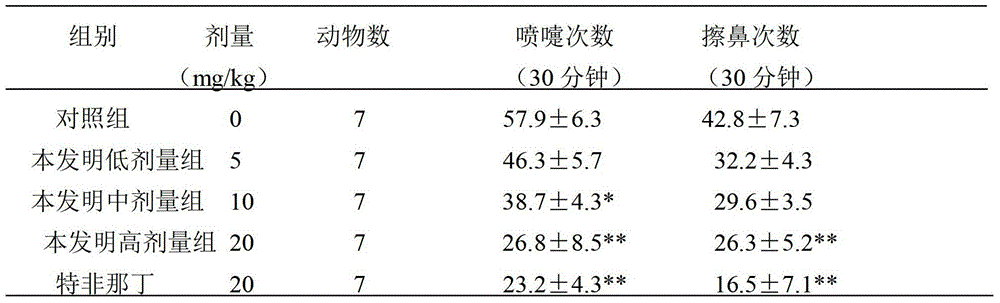
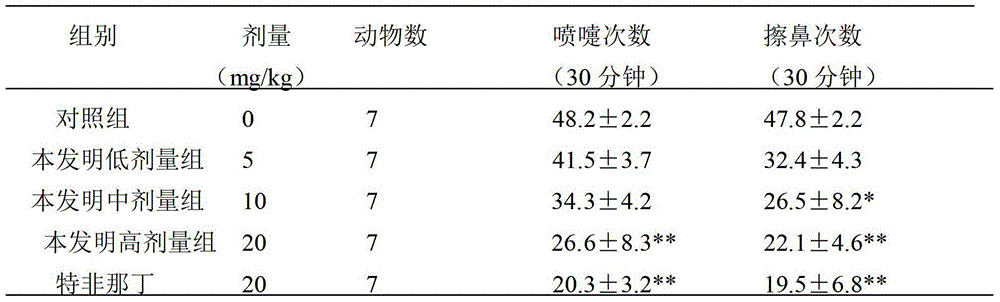
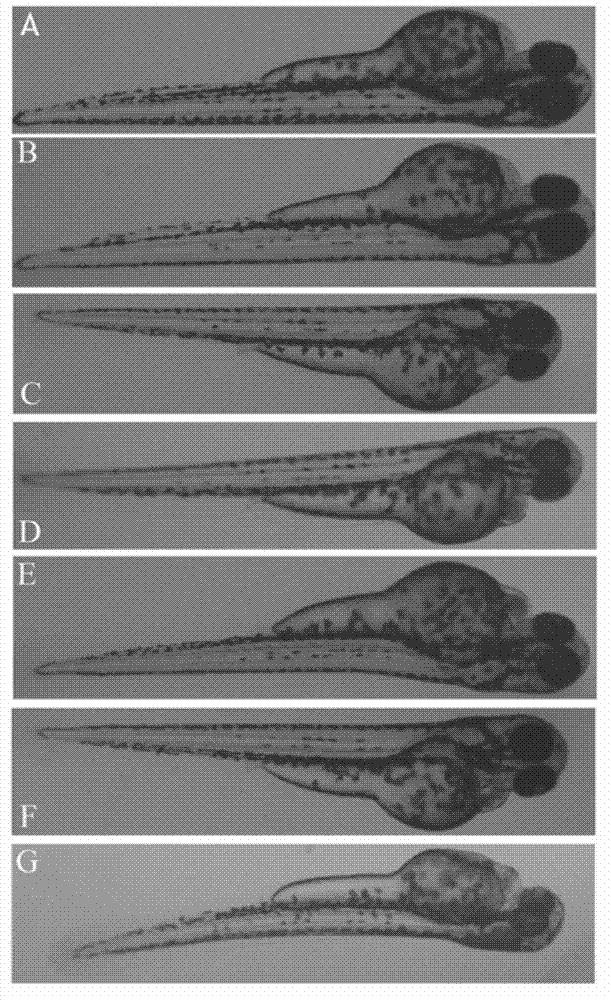
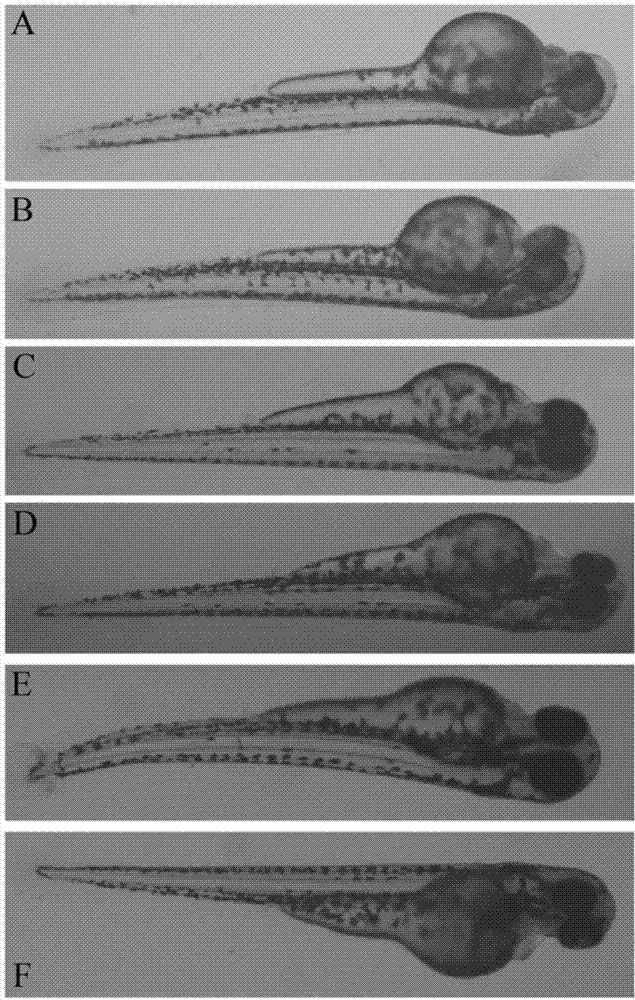
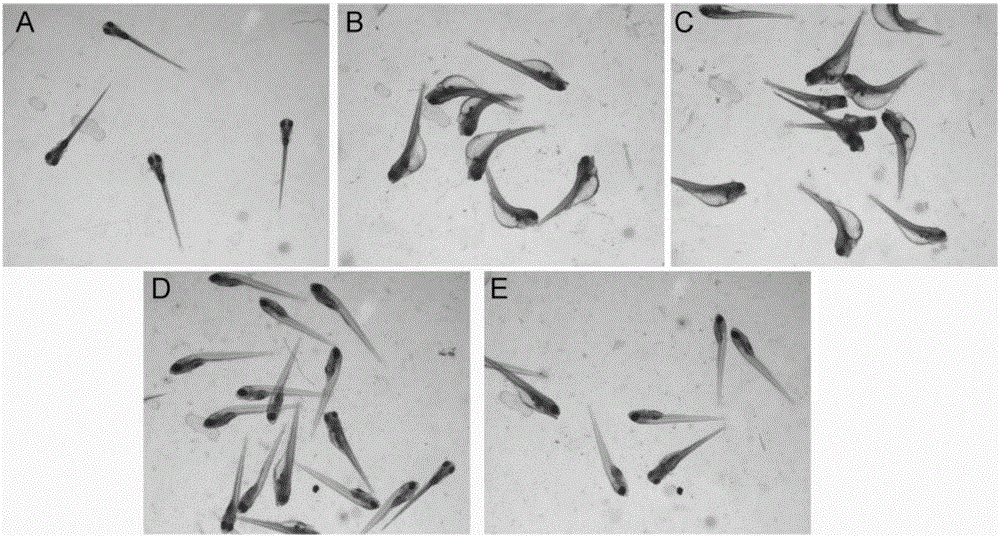
The invention relates to application of terfenadine in preparation of a zebra fish heart function damage model. The application comprises the following steps of putting normally developing zebra fish which is fertilized 2-3 days later into breeding water solution containing terfenadine, and cultivating for 24-48 hours at constant temperature of 28 DEG C to prepare the zebra fish heart function damage model. Terfenadine is utilized for the first time to build the zebra fish heart function damage model; the built zebra fish heart function damage model has the advantages that the zebra fish heart function damage model is simple and convenient to prepare, rapid, stable, reliable, and good in repeatability; the manufacturing cost of the heart function damage model is reduced; and the reliability of the experimental research result is improved.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Application of Myriberine A in preparation of a medicine for treating rhinitis
The invention discloses Myriberine A which is used for treating immunity inflammation, used for preparing a medicine for treating the immunity inflammation, and particularly suitable for preparing a medicine for treating rhinitis, and terfenadine and diclofenac sodium are used as comparison, the curative effect of Myriberine A is clear. An application of Myriberine A in preparation of the medicine for treating rhinitis is firstly disclosed, due to the fact that a skeleton type is brand new, activity for treating rhinitis is strong unexpectedly, the possibility of any inspiration provided by other compounds does not exist, Myriberine A has outstanding substantive feature and obvious improvement for preventing and curing rhinitis.
Owner:张关池
Application of terfenadine in preparation of zebra fish heart function damage model
ActiveCN103230397BEasy to manufactureFast preparationCardiovascular disorderHeterocyclic compound active ingredientsExperimental researchFishery
The invention relates to application of terfenadine in preparation of a zebra fish heart function damage model. The application comprises the following steps of putting normally developing zebra fish which is fertilized 2-3 days later into breeding water solution containing terfenadine, and cultivating for 24-48 hours at constant temperature of 28 DEG C to prepare the zebra fish heart function damage model. Terfenadine is utilized for the first time to build the zebra fish heart function damage model; the built zebra fish heart function damage model has the advantages that the zebra fish heart function damage model is simple and convenient to prepare, rapid, stable, reliable, and good in repeatability; the manufacturing cost of the heart function damage model is reduced; and the reliability of the experimental research result is improved.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Use of Nitrosporeusines A in drugs for treating rhinitis
The invention provides a use of Nitrosporeusines A in preparation of drugs for treating immune-inflammation and especially for treating rhinitis. A contrast test utilizing terfenadine and diclofenac sodium as control groups proves that Nitrosporeusines A has clear effects. Nitrosporeusines A is first disclosed in the invention. Nitrosporeusines A has a novel skeleton type, strong activity of treating rhinitis and substantive distinguishing features, and obviously develops rhinitis prevention and treatment.
Owner:JIANGSU KANGQIANG FOOD LIMITED
Methods and compositions using terfenadine metabolites in combination with leukotriene inhibitors
InactiveUS20030083343A1Reduce and avoid adverse effectSuperior and improved and synergistic effectBiocideSenses disorderMetaboliteDecongestant
Methods and pharmaceutical compositions employing a terfenadine metabolite and a leukotriene inhibitor for the treatment or prevention of inflammation or allergic disorders, such as asthma, or symptoms thereof. Also included are methods and compositions employing a terfenadine metabolite, a leukotriene inhibitor, and a decongestant for the treatment or prevention of inflammation or allergic disorders, such as asthma, or symptoms thereof.
Owner:SUNOVION PHARMA INC
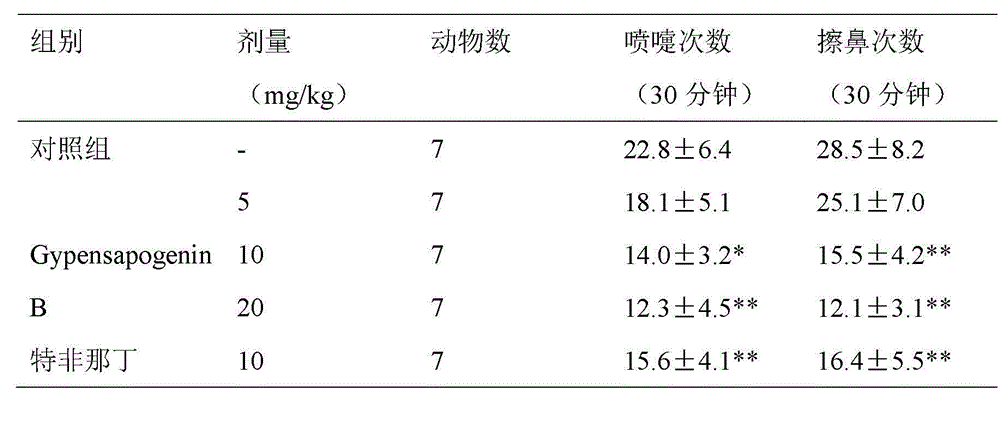
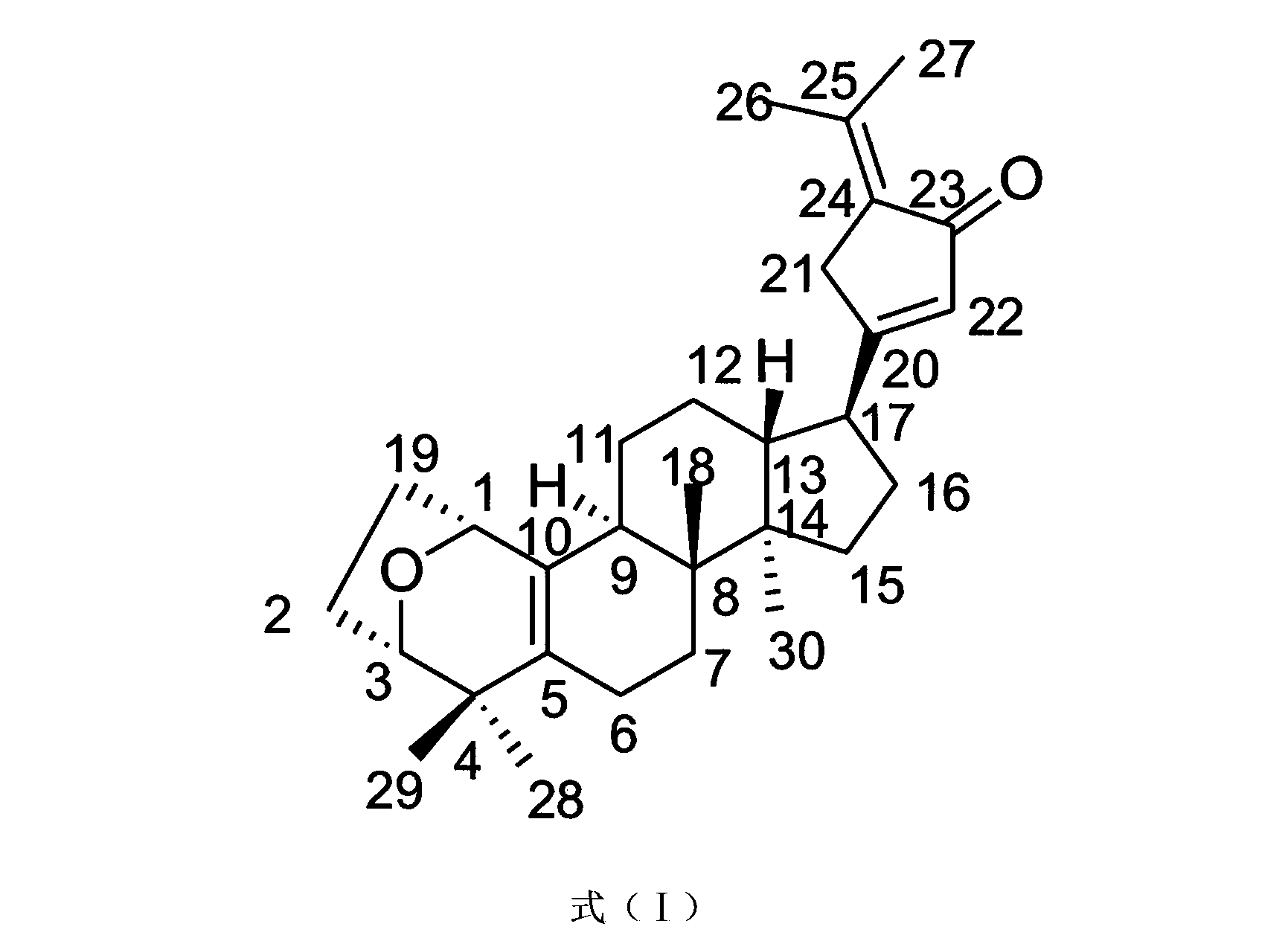
Application of Gypensapogenin B in medicaments for treating rhinitis
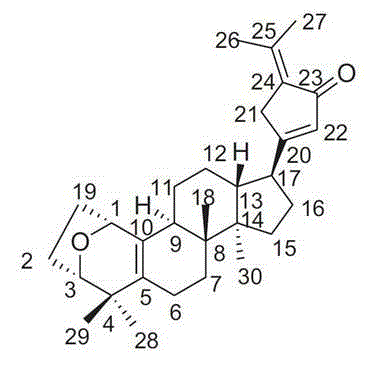
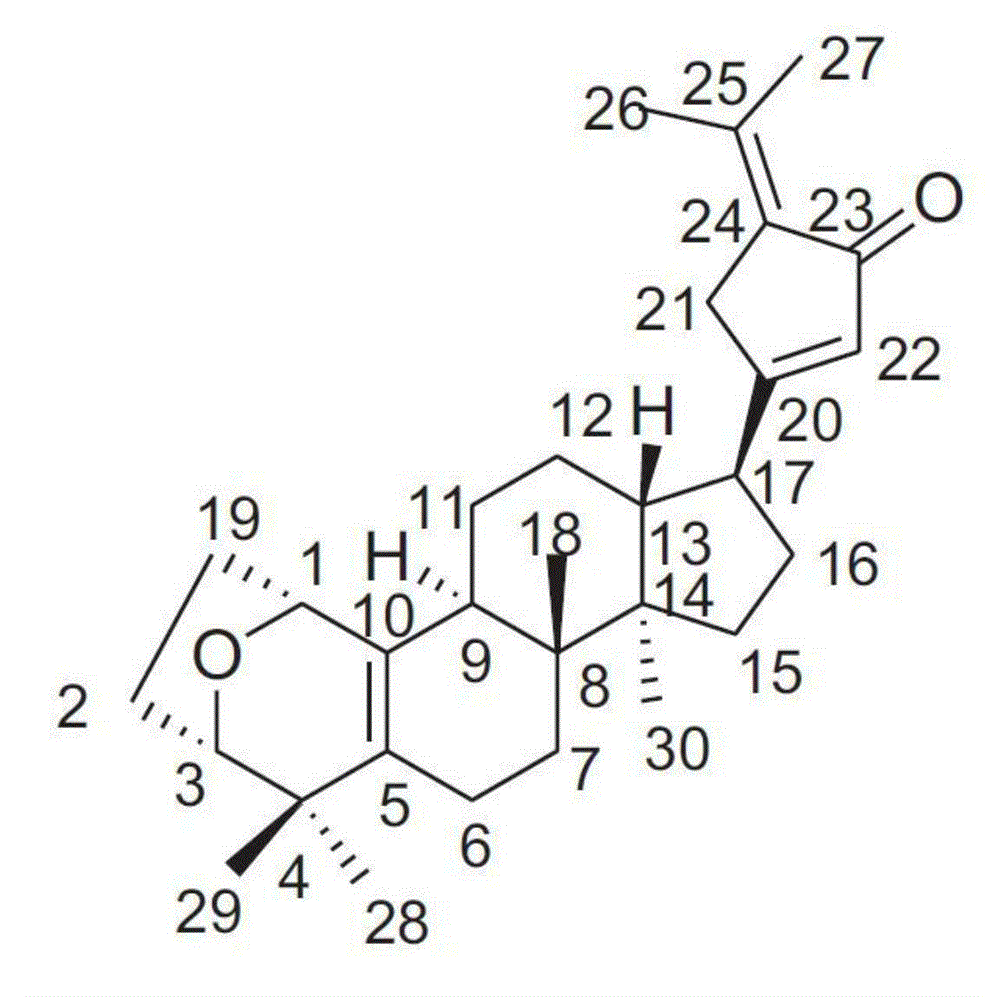
The invention discloses Gypensapogenin B for preparing medicaments for treating immunity inflammation, which is particularly suitable for preparing medicaments for treating rhinitis. By taking Terfenadine and diclofenac sodium as controls, Gypensapogenin B has definite curative effects. The application of Gypensapogenin B in preparing medicaments for treating rhinitis, involved in the invention, is disclosed for the first time. As the skeleton type is brand new, and the strong activity of Gypensapogenin B on treating rhinitis is unexpectedly strong, the possibility that any enlightenments are given by other compounds does not exist. Gypensapogenin B has prominent substantive features and has notable progress when being used for preventing and treating rhinitis.
Owner:JIANGSU KANGHENG CHEM
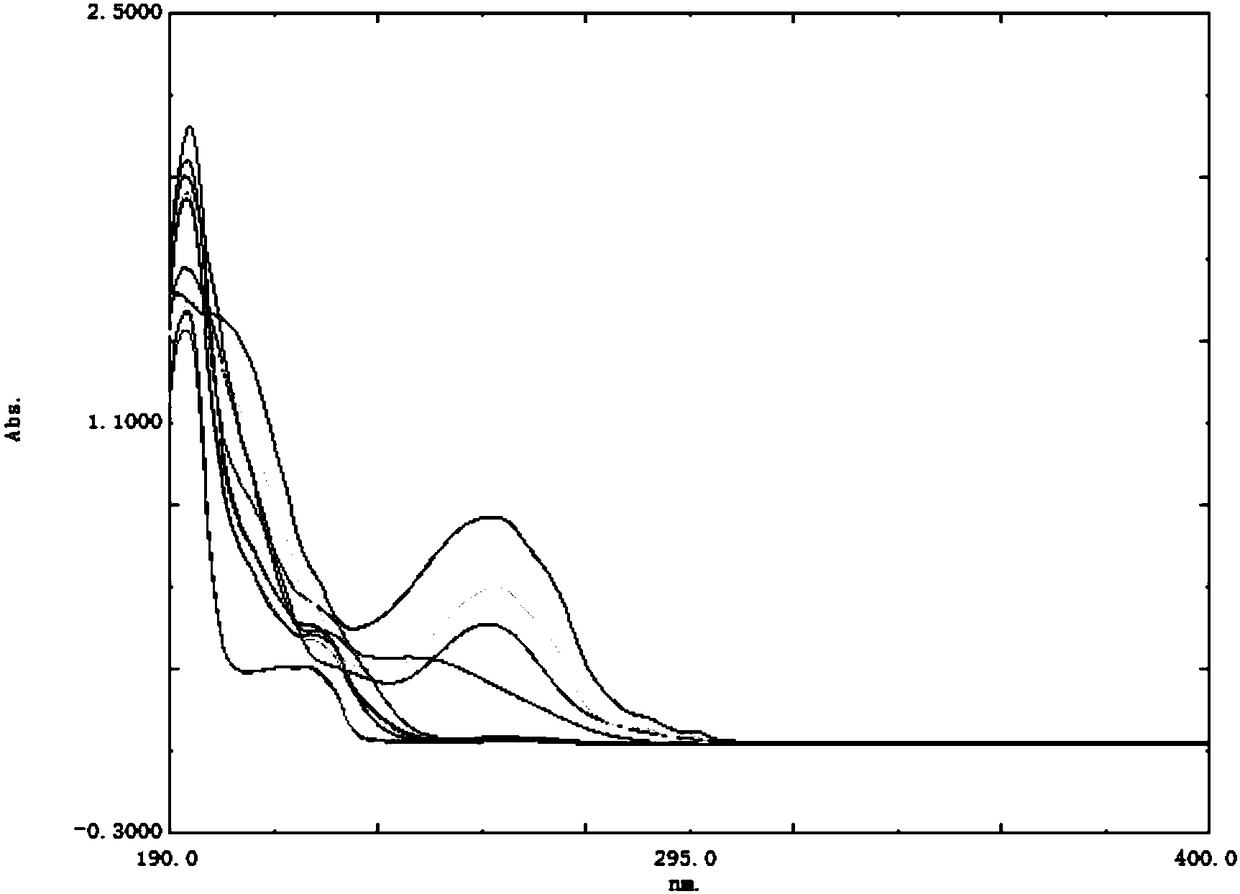
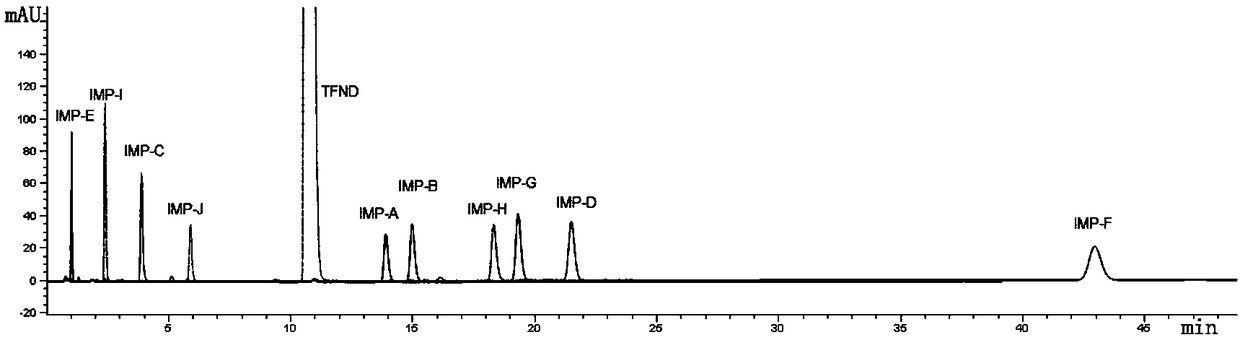
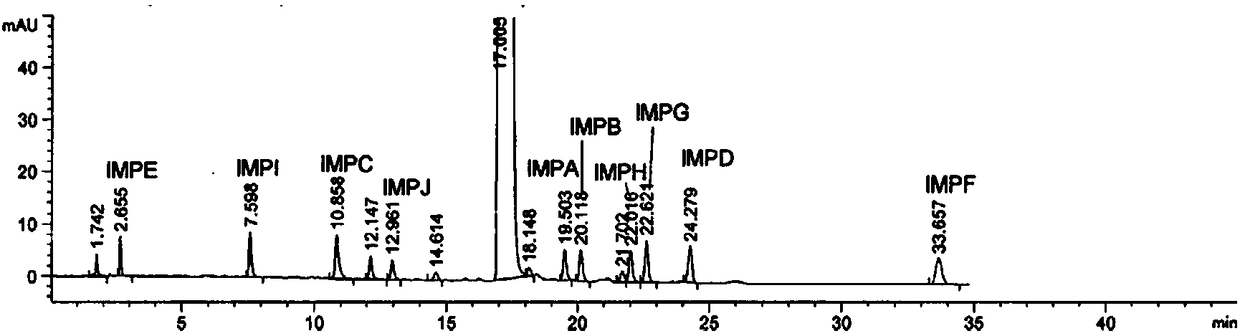
Verification and separation method for terfenadine related substance
The invention discloses a verification and separation method for a terfenadine related substance. The method comprises the following steps: 1) respectively putting 70% acetonitrile into impurities anda proper amount of TFND (Terfenadine) so as to obtain a 10mu g / mL single solution, performing full-wavelength scanning at wavelengths of 190-400nm so as to obtain scanning wavelengths of different substances, and confirming ultraviolet scanning detection wavelengths; 2) preparing a mixed solution for detection; 3) setting the following spectrum conditions: a spectrum column: MZ-NALYTICAL Column of 250*4.6mm 100ODS 5mu m; a mobile phase A: acetonitrile and phosphate in a ratio of 20:80; a mobile phase B: acetonitrile; gradient elution: 0 minute, 60%A, 40% B, 3 minutes, 55%A, 45%B, 20 minutes,20% A and 80%B; a solvent of acetonitrile and phosphate in a ratio of 45:55; a wavelength of 217nm; a flowing velocity of 1.0mL / minute; a column temperature of 30-35 DEG C; a sampling amount of 20mu L; 4) feeding the mixed solution as a sample, recording chromatogram, and separating both terfenadine and different impurities. By adopting the method, the separation degree of the terfenadine and theimpurities is greater than 5.0, the separation degrees among the impurities and between other known impurities and the terfenadine are both greater than 1.5, in addition, the separation degree of a main peak and unknown impurities can be up to 3.5, and requirements can be met.
Owner:JIANGSU LIANHUAN PHARMA
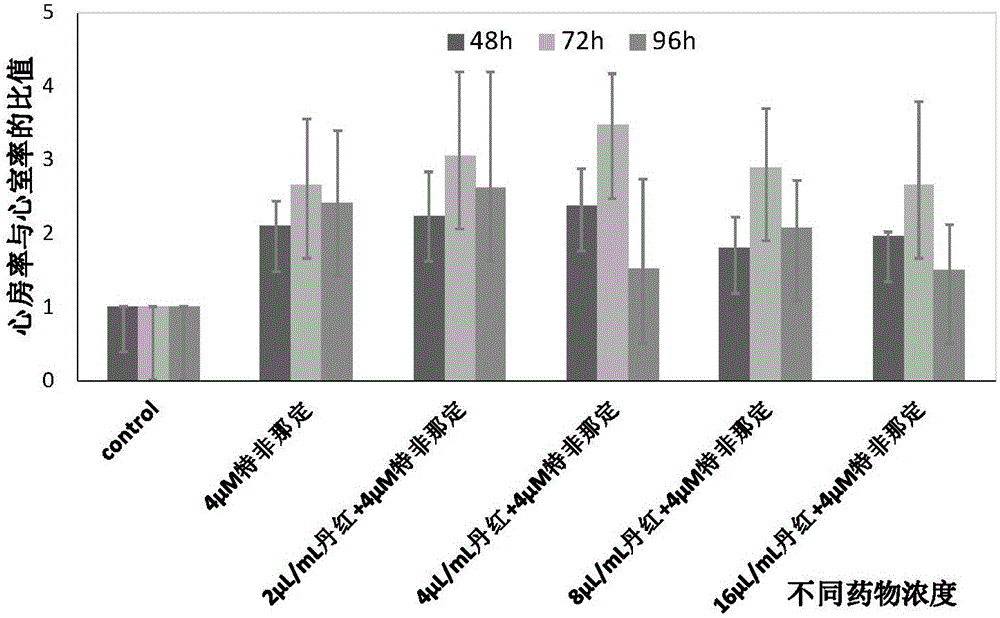
Application of Danhong injection as terfenadine heart adverse effect antagonist
InactiveCN105943605AReduce adverse reactionsImproved heart rhythm changesPharmaceutical delivery mechanismCardiovascular disorderTerfenadineAntagonist
The invention discloses an application of a Danhong injection as a terfenadine heart adverse effect antagonist. The application overcomes the technology bias of the prior art on the aspect of application, and experiments show that the Danhong injection has a remarkable effect of improving the heart rhythm change, caused by terfenadine, of a zebra fish. In addition, when the concentration of the Danhong injection is less than or equal to 30muL / mL, the normal zebra fish does not show an obvious teratogenic effect after 6 days of continuous injection. On the basis of the extensive clinical application of the Danhong injection, a theoretical foundation is provided for the drug combination of the terfenadine and the enhancement of the safety of the terfenadine.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Methods and compositions using racemic, (r)-, and (s)-fexofenadine in combination with leukotriene inhibitors
InactiveUS20120149730A1Potent antihistaminic activityConvenient treatmentBiocideSenses disorderFexofenadineMetabolite
Methods and pharmaceutical compositions employing a terfenadine metabolite and a leukotriene inhibitor for the treatment or prevention of inflammation or allergic disorders, such as asthma, or symptoms thereof. Also included are methods and compositions employing a terfenadine metabolite, a leukotriene inhibitor, and a decongestant for the treatment or prevention of inflammation or allergic disorders, such as asthma, or symptoms thereof.
Owner:SUNOVION PHARMA INC
Application of racemosins A in preparation of medicine treating rhinitis
Racemosins A treating immune inflammation is used for preparing the medicine treating the immune inflammation and is especially suitable for preparing the medicine treating the rhinitis. Compared with terfenadine and diclofenac sodium, the racemosins A is good in therapeutic effect. The application of the racemosins A in preparation of the medicine treating the rhinitis is disclosed for the first time. The matrix type of the racemosins A belongs to a brand new matrix type. The racemosins A has the advantages of being high in activity for treating the rhinitis and prominent in substantive features, and makes remarkable progress in preventing and treating the rhinitis.
Owner:段仲达
Application of biscarpamontamine A in preparation of rhinitis pharmaceuticals
Biscarpamontamine A for treating immunity inflammation is used for preparing pharmaceuticals for treating immunity inflammation and particularly suitable for preparing pharmaceuticals for treating rhinitis. Terfenadine and Diclofenac are used as controls, the biscarpamontamine A has exact curative effect and is disclosed for the first time, and as a frame type is a brand-new frame type, having high rhinitis treatment activity, the biscarpamontamine A has excellent substantive features and has significant progress in rhinitis prevention and treatment.
Owner:ZIBO DINGLI PATENT INFORMATION CONSULTING CO LTD
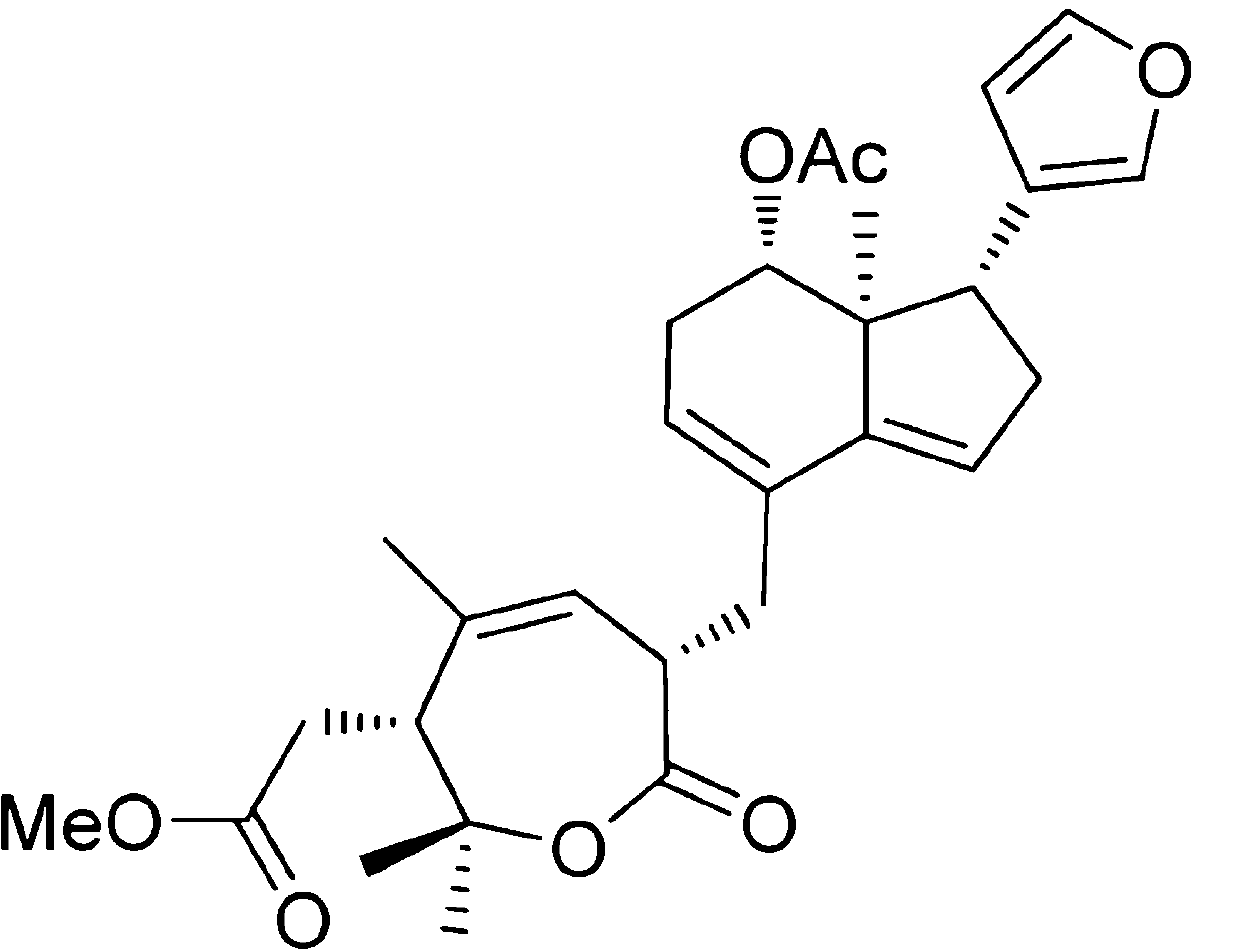
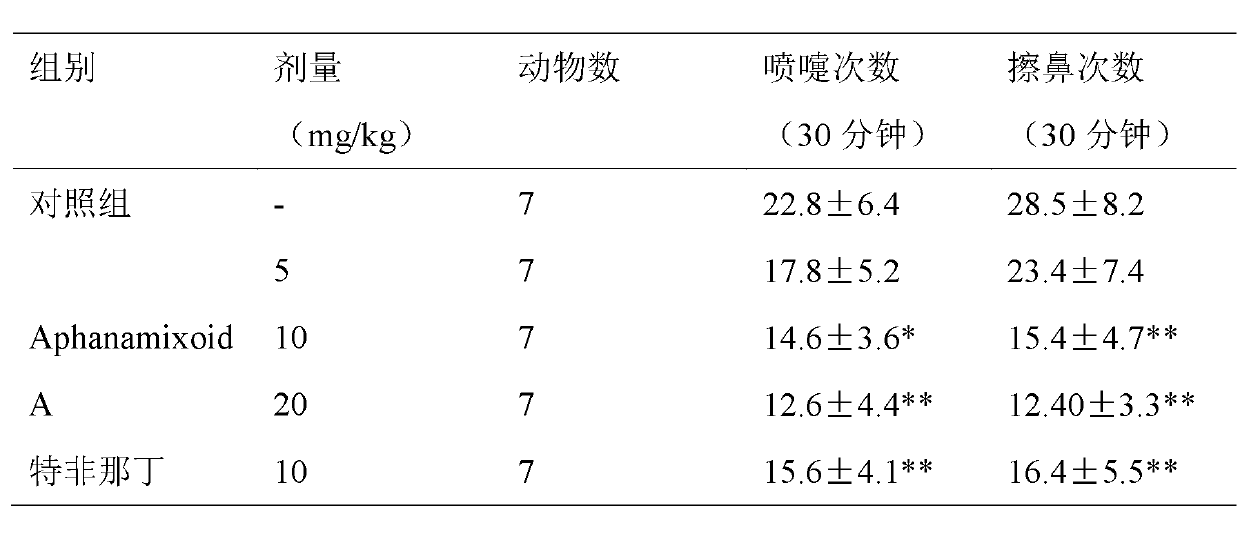
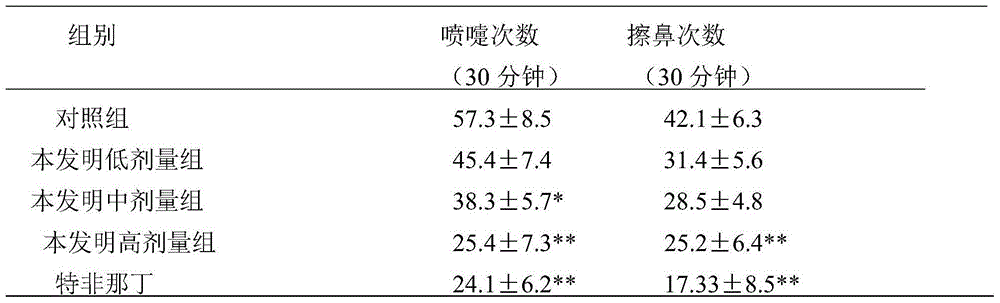
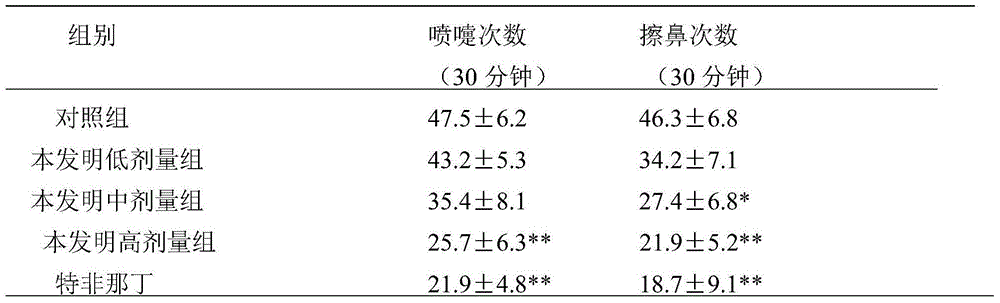
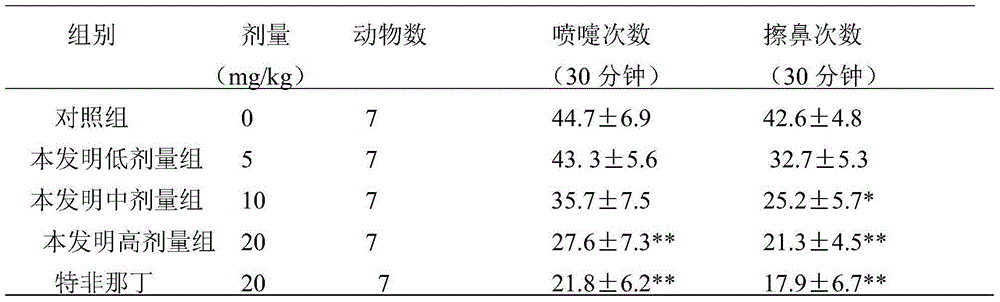
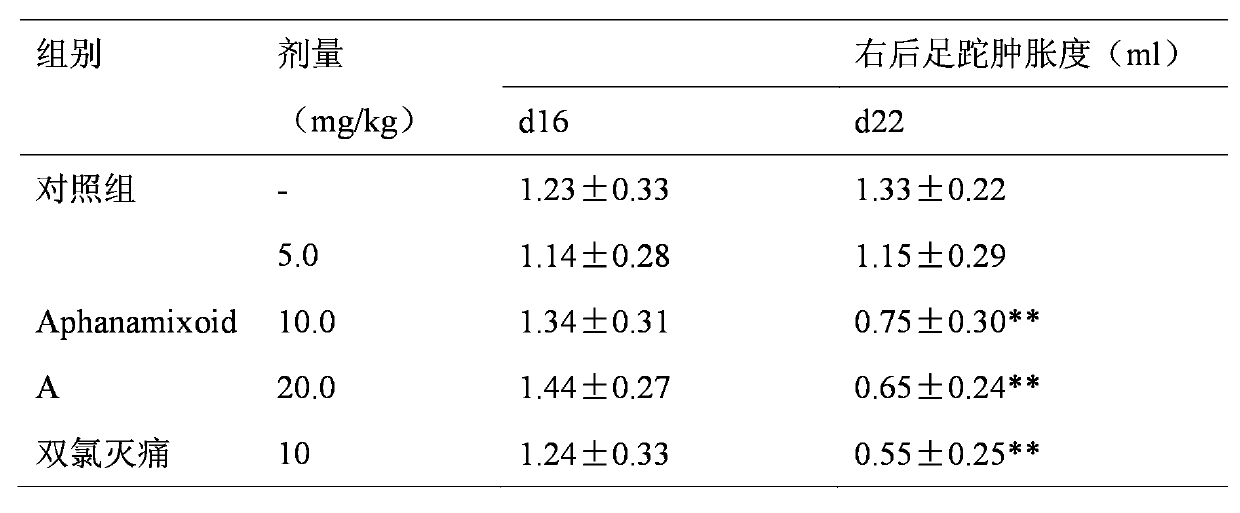
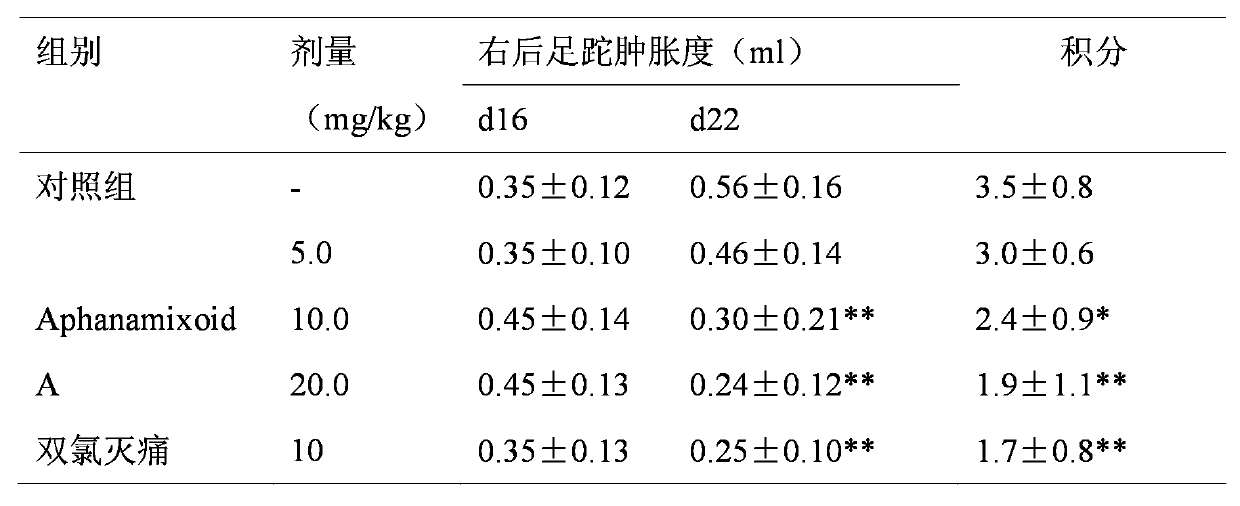
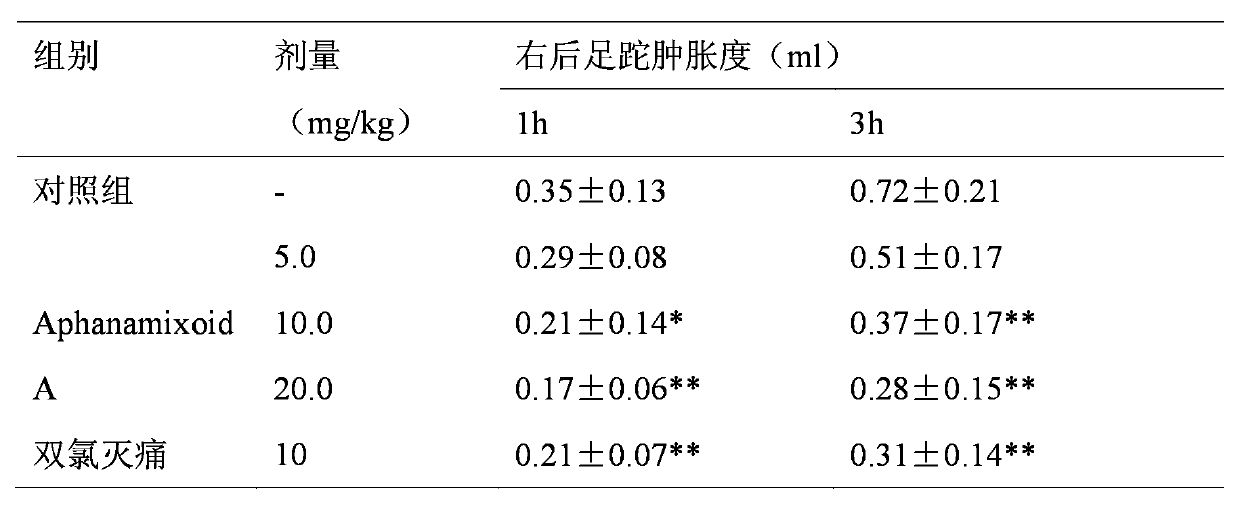
Application of Aphanamixoid A to rhinitis treatment medicine
InactiveCN103127078ASignificant progressStrong activity in treating rhinitisOrganic active ingredientsRespiratory disorderDiclofenac SodiumCurative effect
Aphanamixoid A for treating immunity inflammation is used for preparing immunity inflammation treatment medicine and particularly suitable for preparing rhinitis treatment medicine. Compared with terfenadine and diclofenac sodium, the Aphanamixoid A is obvious in curative effect. The application of the Aphanamixoid A to preparation of the rhinitis treatment medicine is made public for the first time. The skeleton type is fully novel, activity of the Aphanamixoid A in treating rhinitis is surprisingly strong, the possibility that inspiration is obtained from other compounds does not exist, and therefore the Aphanamixoid A has prominent substantive features, and meanwhile, significant progress of the application of the Aphanamixoid A to prevention and treatment of the rhinitis is obviously achieved.
Owner:吴俊华
Application of traditional Chinese medicinal composition in preparation of rhinitis treatment medicines
InactiveCN104825813ASignificant progressHighlight substantive featuresRespiratory disorderPlant ingredientsGymnema latifoliumCurative effect
A traditional Chinese medicinal composition for treating immune inflammations is prepared from 15 parts of Ford Nervilia Leaf, 12 parts of root of Dentiferous Dendropanax, 18 parts of Gymnema latifolium, 10 parts of Zanthoxylum ailanthoides Sieb. et Zucc. and 20 parts of Sowthistleleaf Tasselflower. The composition is used for preparing immune inflammation treatment medicines, and is especially suitable for preparing rhinitis treatment medicines. Compared with terfenadine and diclofenac sodium, the traditional Chinese medicinal composition has the advantages of clear curative effect and marked progress.
Owner:ZIBO DINGLI PATENT INFORMATION CONSULTING CO LTD
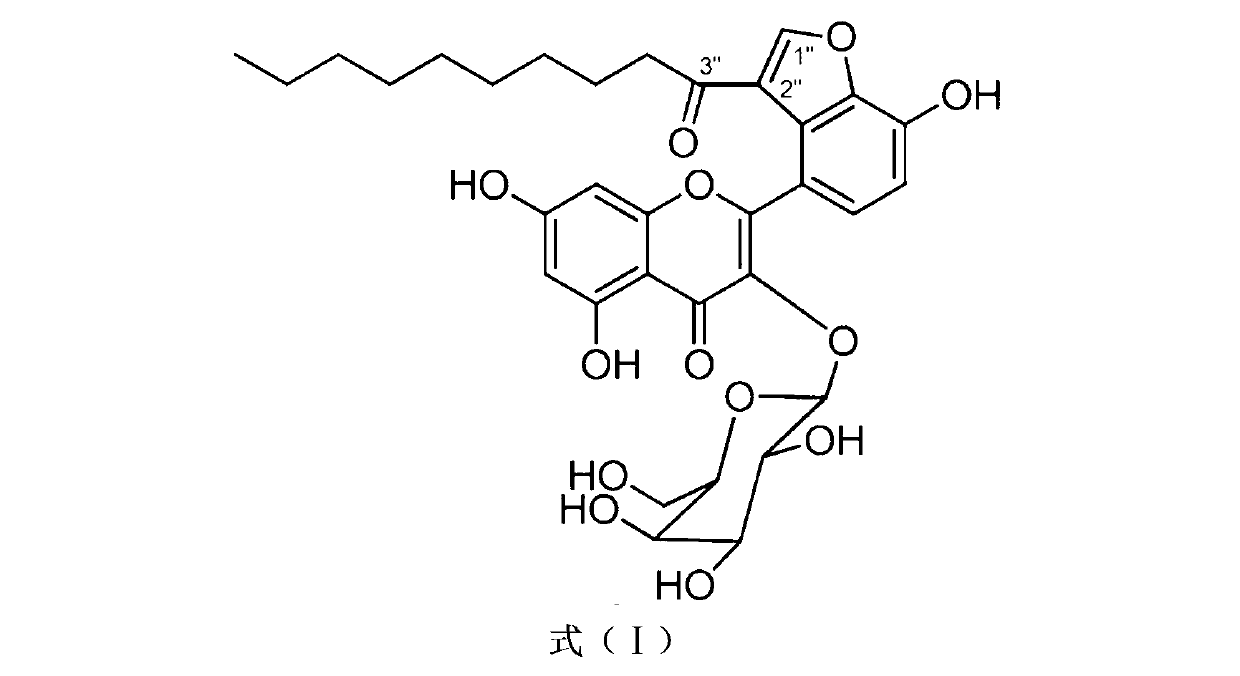
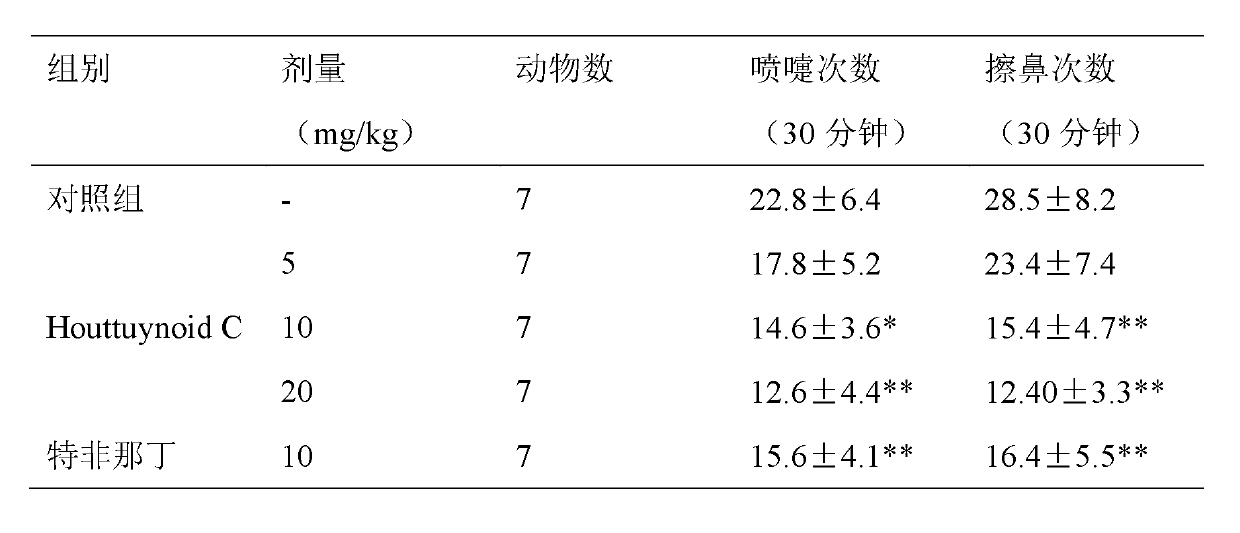
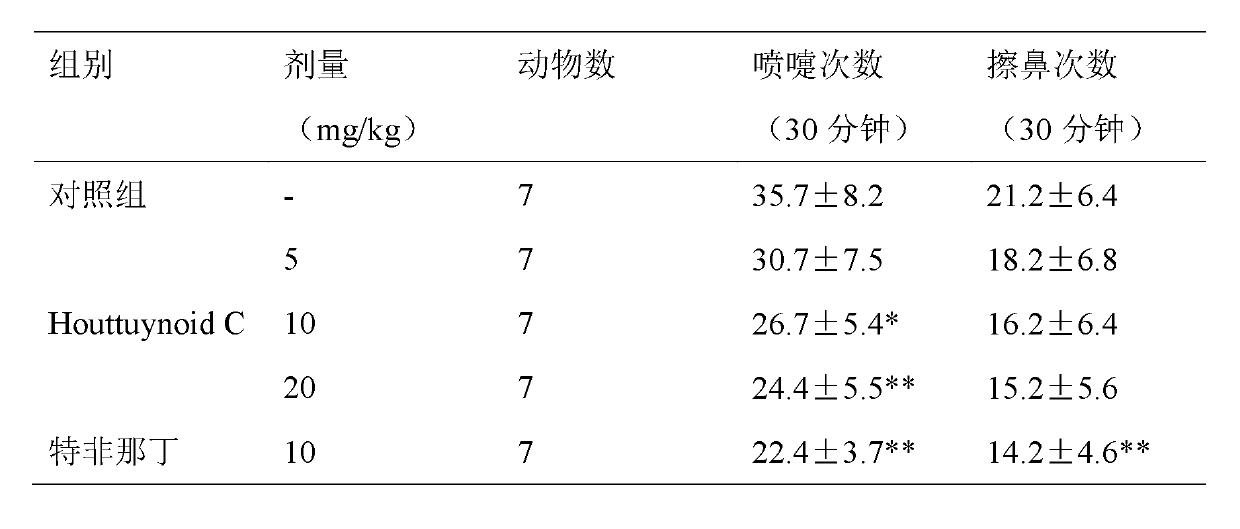
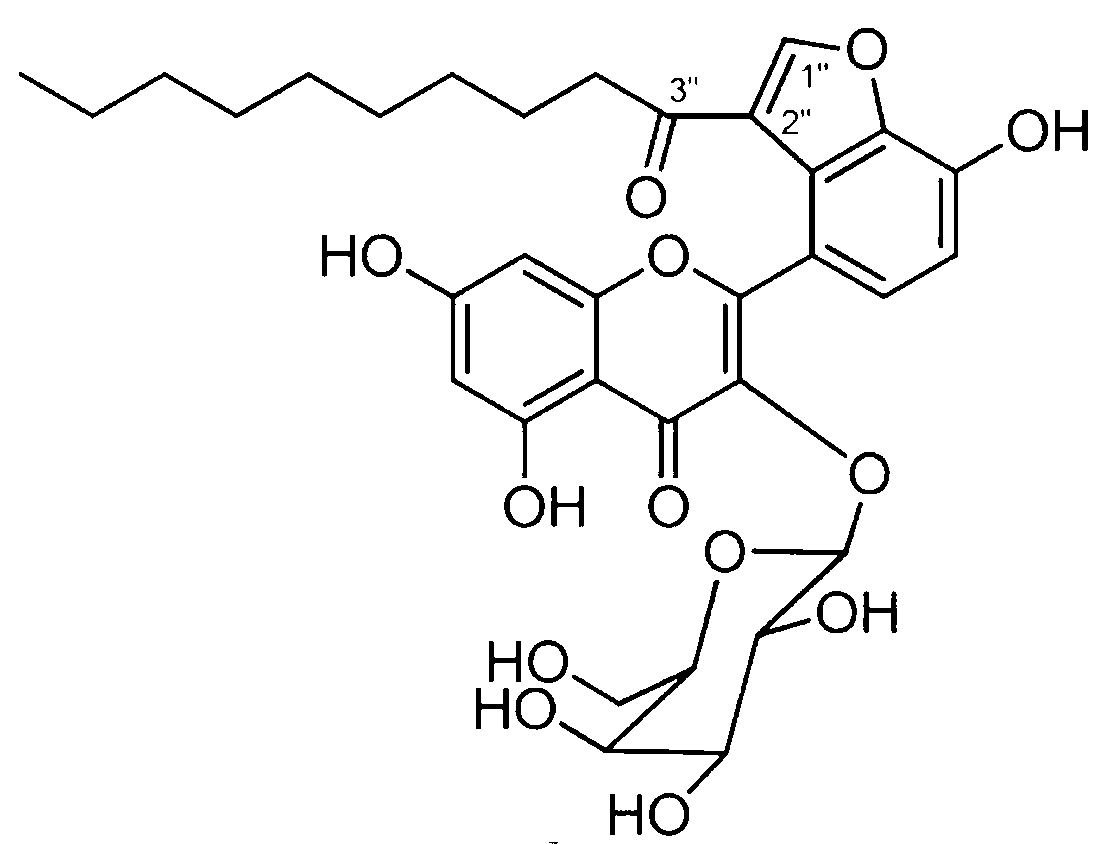
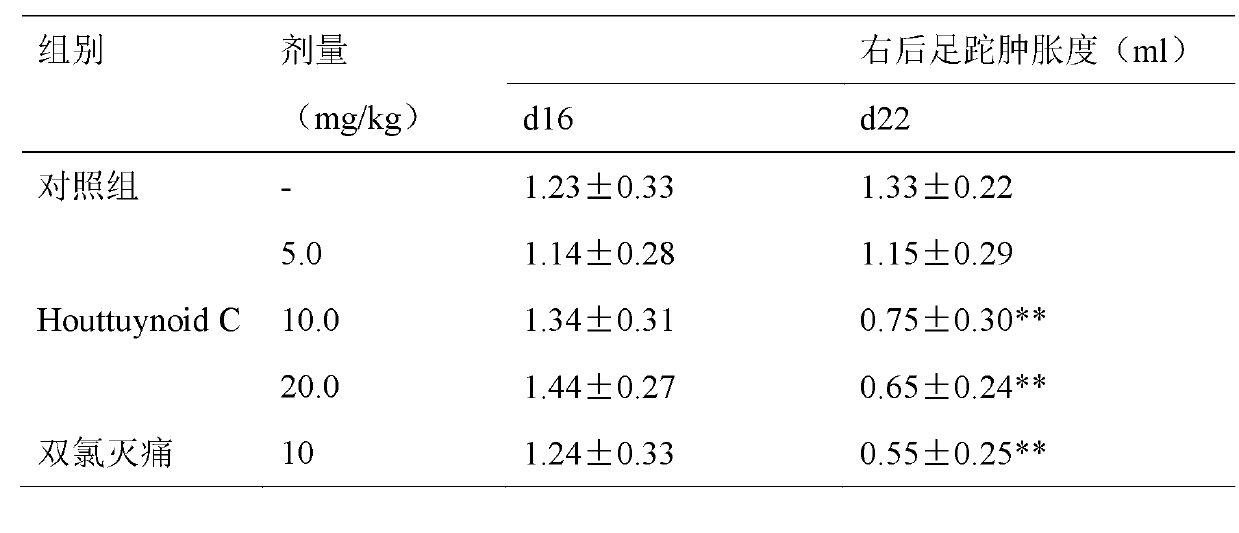
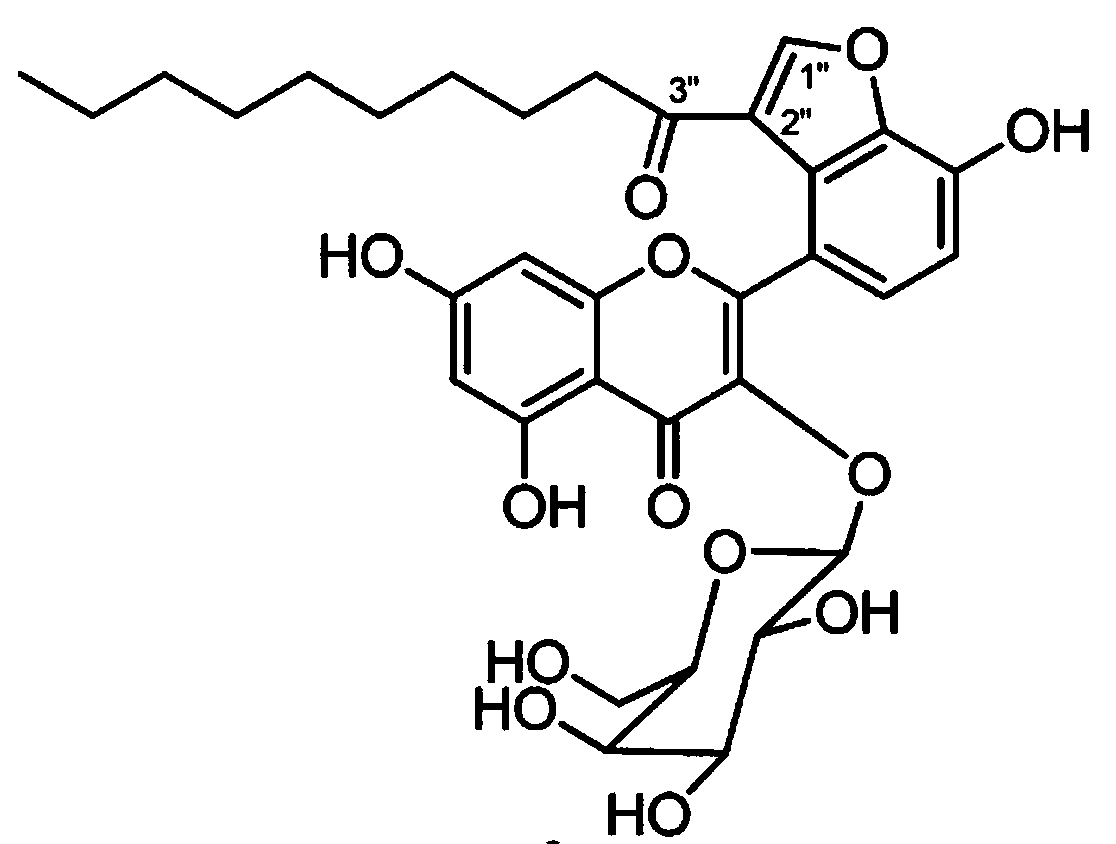
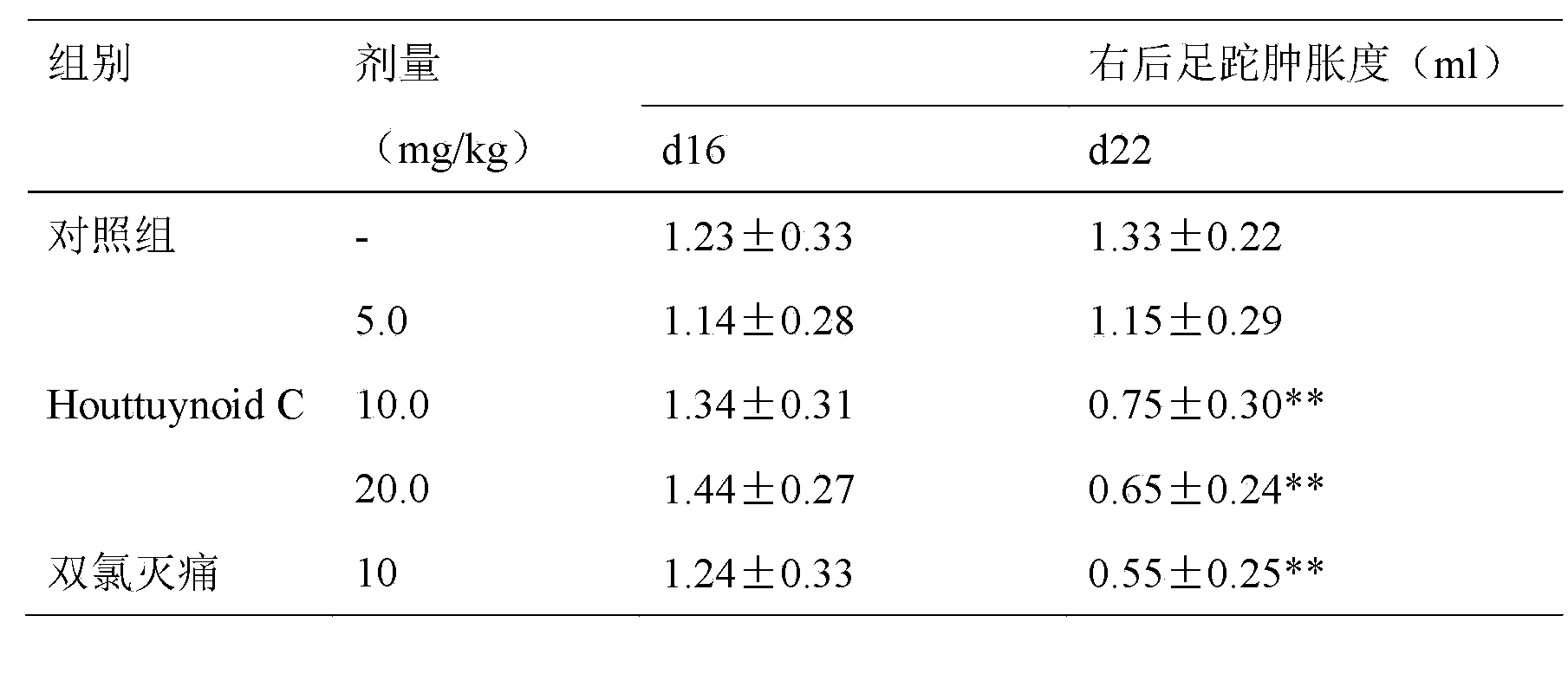
Application of Houttuynoid C for preparing medicine for treating rhinitis
The invention relates to Houttuynoid C for treating immunity inflammation, which is used for preparing a medicine for treating immunity inflammation, in particular for preparing a medicine for treating rhinitis. Houttuynoid C has definite curative effect by using terfenadine and diclofenac sodium as contrasts. The application of Houttuynoid C for preparing the medicine for treating rhinitis is disclosed for the first time; because the skeleton type belongs to a brand new skeleton type and the rhinitis curative activity of Houttuynoid C is strong unexpectedly, the possibility that other compounds give any inspiration does not exist; Houttuynoid C has outstanding substantive characteristics; and simultaneously, Houttuynoid C has obvious progress for preventing and treating rhinitis.
Owner:何晓涛
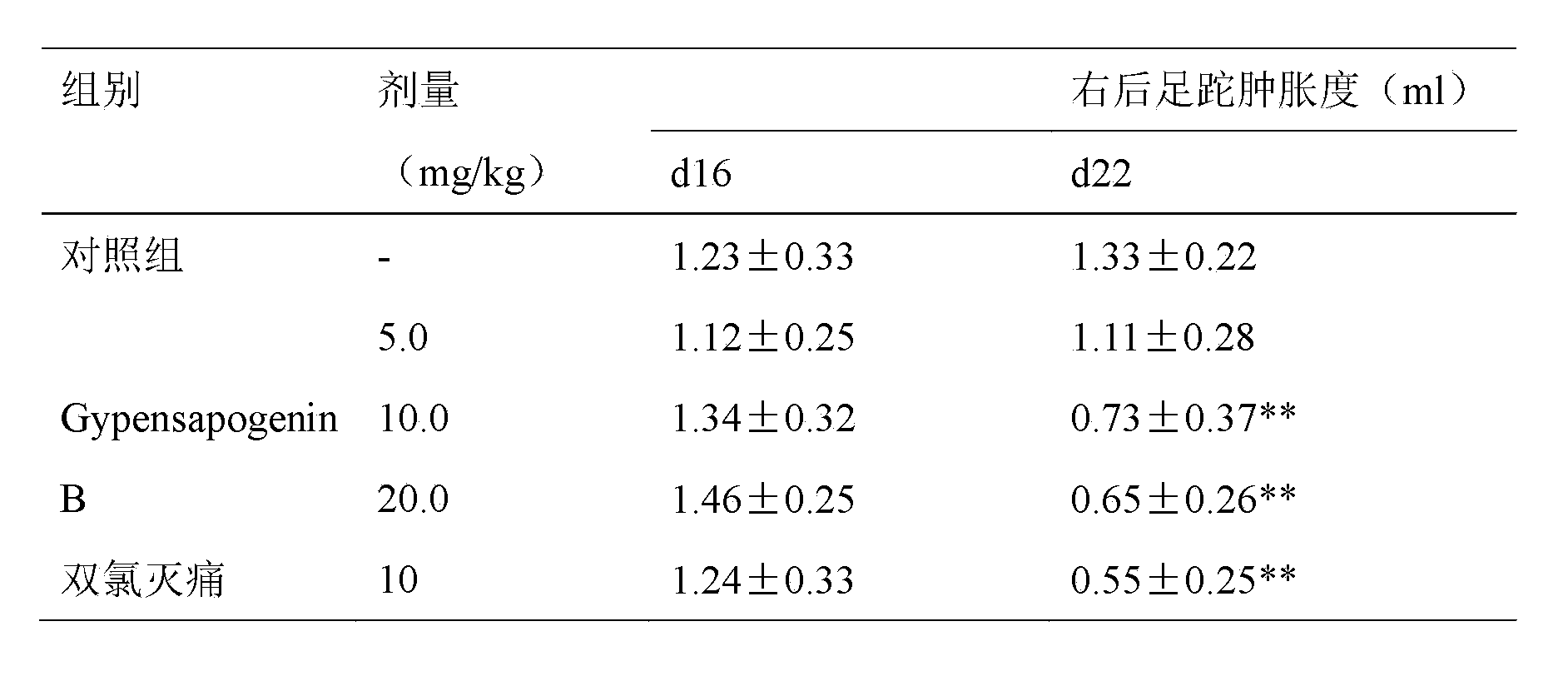
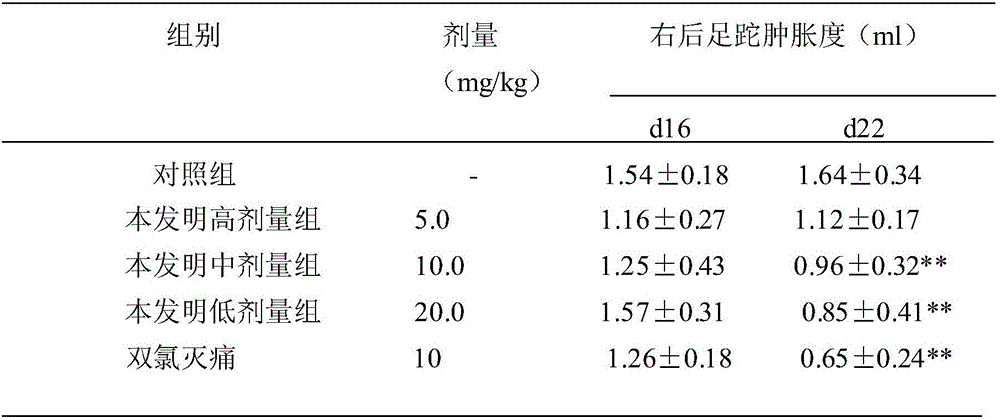
Application of Gypensapogenin B in medicament for treating rheumatoid arthritis
ActiveCN102885828BStrong inhibitory activitySignificant progressOrganic active ingredientsAntipyreticArthritis- rheumatoid arthritisDiclofenac Sodium
The invention relates to Gypensapogenin B for treating immune inflammation and preparing a medicament for treating rheumatoid arthritis. By using terfenadine and diclofenac sodium as contrasts, the Gypensapogenin B has definite treatment effect. The application of the Gypensapogenin B in preparation of the medicament for treating rheumatoid arthritis is disclosed for the first time, the skeleton type is a brand-new skeleton type, the Gypensapogenin B has unexpected strong inhibiting activity on the rheumatoid arthritis, the probability of giving any revelation by other compounds does not exit, and the Gypensapogenin B has predominant substantial characteristics and obviously has a remarkable progress in prevention and treatment of the rheumatoid arthritis.
Owner:启东市天汾电动工具技术创新中心
Application of Linderolide H in preparation of drugs for treating rheumatoid arthritis
InactiveCN106344560AStrong inhibitory activitySignificant progressOrganic active ingredientsSkeletal disorderCurative effectTherapeutic effect
Linderolide H for treating immune inflammations is used to prepare drugs for treating rheumatoid arthritis; by using terfenadine and diclofenac as controls, Linderolide H has exact treatment effect; the application of Linderolide H related herein in the preparation of drugs for treating rheumatoid arthritis is disclosed for the first time; since a framework is of brand-new type and the inhibitory activity for rheumatoid arthritis is unexpectedly high, there is no chance for other compounds to give any indication and outstanding practical characteristics are provided; in addition, Linderolide H has significant progress in preventing and treating rheumatoid arthritis.
Owner:ZIBO DINGLI PATENT INFORMATION CONSULTING CO LTD
Application of marine phospholipids as active ingredients in the preparation of drugs for preventing and/or treating heart disease
ActiveCN112494499BImprove protectionGood treatment effectOrganic active ingredientsCrustacean material medical ingredientsDiseaseThrombus
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
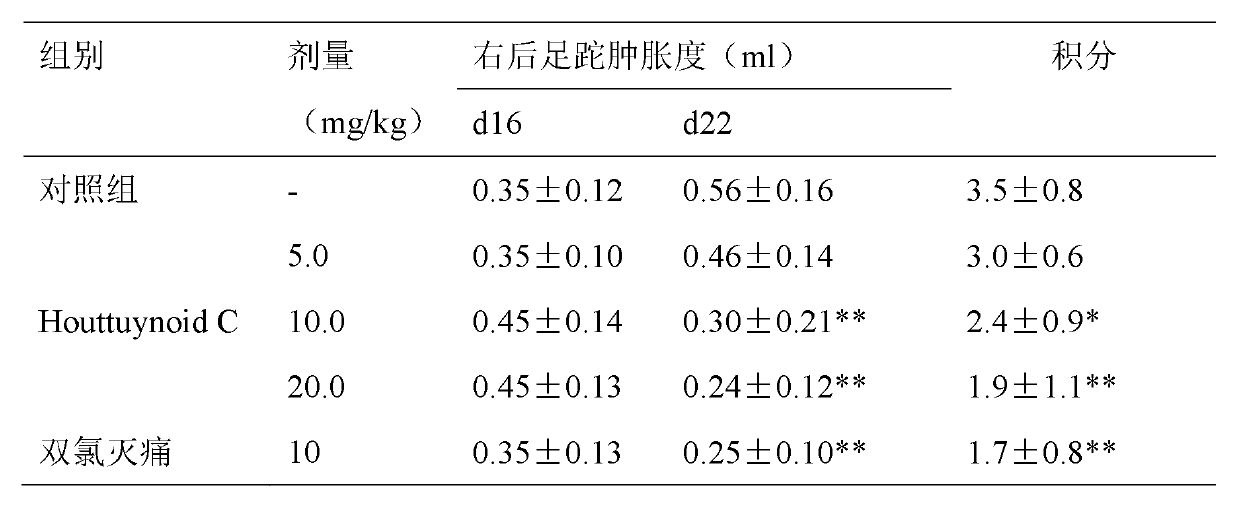
Application of Houttuynoid C in preparation of medicine for treating rheumatoid arthritis
InactiveCN102988378AInhibits the writhing responseInhibit acute and chronic inflammationOrganic active ingredientsAntipyreticArthritis- rheumatoid arthritisDiclofenac Sodium
Owner:何晓涛
Application of Sphenostylisins C in preparation of drugs used for treating rhinitis
The invention provides Sphenostylisins C used for treating immunity inflammation. The Sphenostylisins C is applicable to preparation of immunity inflammation drugs, especially preparation of drugs used for treating rhinitis. In contrast to terfenadine and diclofenac sodium, the Sphenostylisins C has definite curative effect. The application of the Sphenostylisins C in preparation of drugs used for treating rhinitis is disclosed for the first time; since the skeleton type of the Sphenostylisins C is a brand new skeleton type and good activity of the Sphenostylisins C in treatment of rhinitis is unexpected, possibility of inspiration by any other compound does not exist and the Sphenostylisins C has protruding substantive distinguishing features; meanwhile, substantial progress is made as the Sphenostylisins C is applied to prevention and treatment of rhinitis.
Owner:ZIBO DINGLI PATENT INFORMATION CONSULTING CO LTD
Use of Nitrosporeusines A in drugs for treating rhinitis
The invention provides a use of Nitrosporeusines A in preparation of drugs for treating immune-inflammation and especially for treating rhinitis. A contrast test utilizing terfenadine and diclofenac sodium as control groups proves that Nitrosporeusines A has clear effects. Nitrosporeusines A is first disclosed in the invention. Nitrosporeusines A has a novel skeleton type, strong activity of treating rhinitis and substantive distinguishing features, and obviously develops rhinitis prevention and treatment.
Owner:JIANGSU KANGQIANG FOOD LIMITED
Application of Aphanamixoid A in medicine for treating rheumatoid arthritis
InactiveCN103120671AInhibits the writhing responseInhibition of primaryOrganic active ingredientsAntipyreticArthritis- rheumatoid arthritisCurative effect
The invention discloses Aphanamixoid A for treating immunity inflammation, and particularly relates to Aphanamixoid A for preparing a medicine for treating rheumatoid arthritis. The rheumatoid arthritis is definite in curative effect by taking terfenadine and diclofenac sodium as references. The application of the Aphanamixoid A in preparation of the medicine for treating the rheumatoid arthritis is firstly disclosed because a skeleton type belongs to a fire-new skeleton type and the inhibitory activity of the Aphanamixoid A on the rheumatoid arthritis is inconceivably high; and the Aphanamixoid A has outstanding substantiality characteristic without possibility of any inspiration given by other compounds and obviously achieves the outstanding progress by being used for preventing and treating the rheumatoid arthritis.
Owner:吴俊华
Novel composition for treating psoriasis
InactiveCN112641923ALow priceSimple preparation processPeptide/protein ingredientsCarbohydrate active ingredientsInflammatory factorsVitamin C
The invention discloses a novel composition for treating psoriasis. The composition comprises the following components of, in parts by weight, 0.005-0.02 parts of arotinoid ethylester, 1-6 parts of polypeptide, 0.07-0.27 parts of ribose, 15-60 parts of terfenadine, 50-200 parts of vitamin E, 500-2000 parts of aminopeptide, 50-200 parts of aminophylline, 1-10 parts of chlorpheniramine maleate, 100-400 parts of vitamin C, 3-12 parts, 1.5-10 parts of vitamin B2, 10-40 parts of tripterygium glycosides, 4.58-18.33 parts of microcrystalline cellulose, 3.33-13.33 parts of pregelatinized starch, 6.67-26.67 parts of polyvinylpolypyrrolidone and 1-4 parts of talcum powder. The invention provides a method for treating psoriasis by combining traditional Chinese medicines and western medicines. Based on gene regulation and transfer termination of inflammatory factors, cellular immunity and humoral immunity are enhanced at the same time, and the purpose of treating both symptoms and root causes is finally achieved.
Owner:烟台心舒医药科技有限公司
Application of Nardoaristolones A in preparing medicines for treating rheumatoid arthritis
ActiveCN103393635BStrong inhibitory activitySignificant progressOrganic active ingredientsAntipyreticMedicineCurative effect
The invention relates to Nardoaristolones A for treating immune inflammation and the Nardoaristolones can be used for preparing medicines for treating rheumatoid arthritis. Terfenadine and diclofenac sodium are taken as control, and the curative effect of Nardoaristolones A is exact. The use of Nardoaristolones A for preparing medicines for treating rheumatoid arthritis provided by the invention is disclosed for the first time. As the framework type belongs to a brand-new framework type, Nardoaristolones A has remarkable substantive characteristics and obviously has remarkable progress for preventing and treating rheumatoid arthritis.
Owner:SHANGHAI STEPPHARM CO LTD
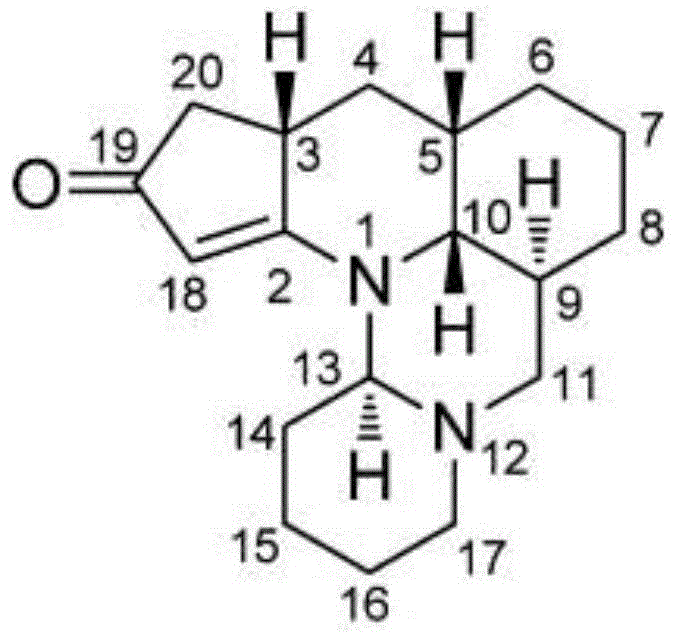
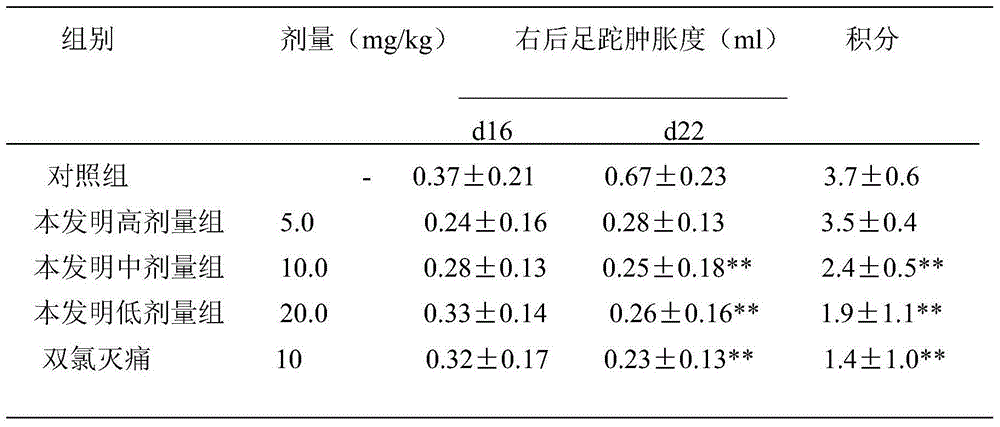
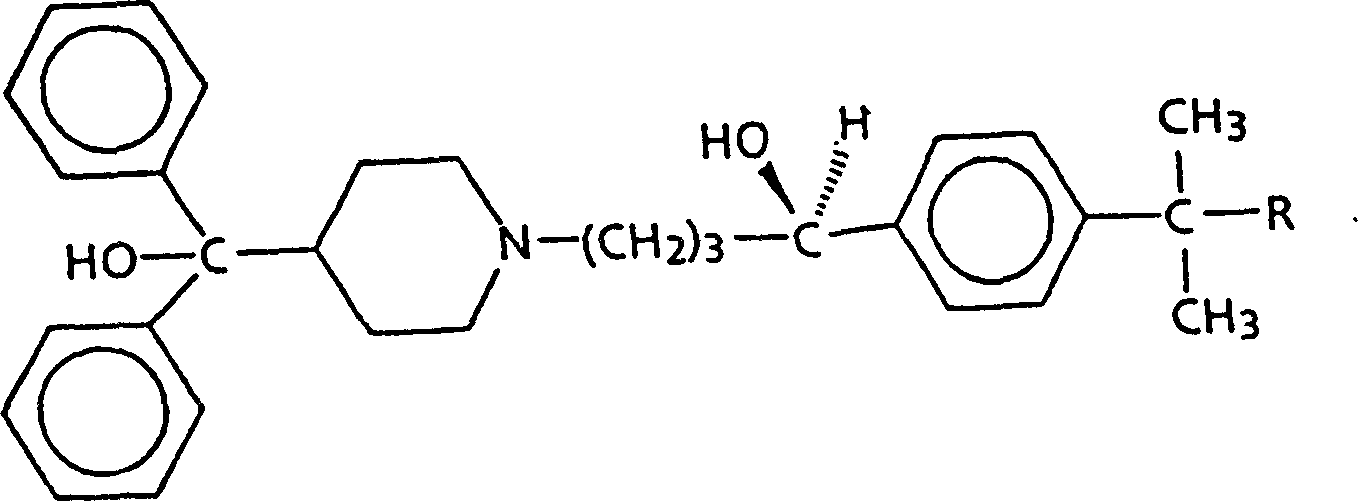
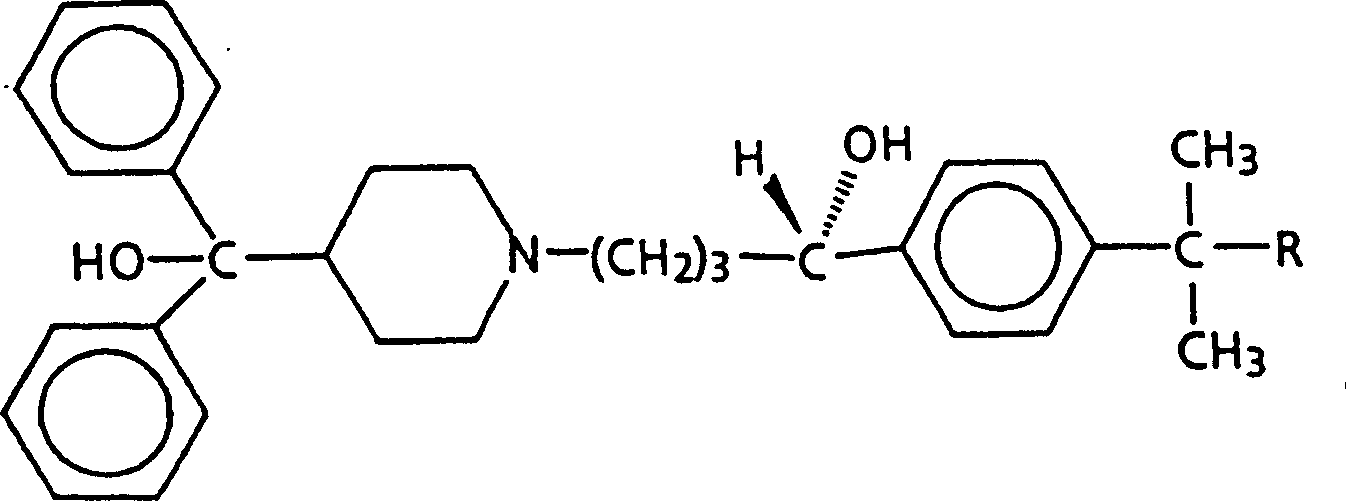
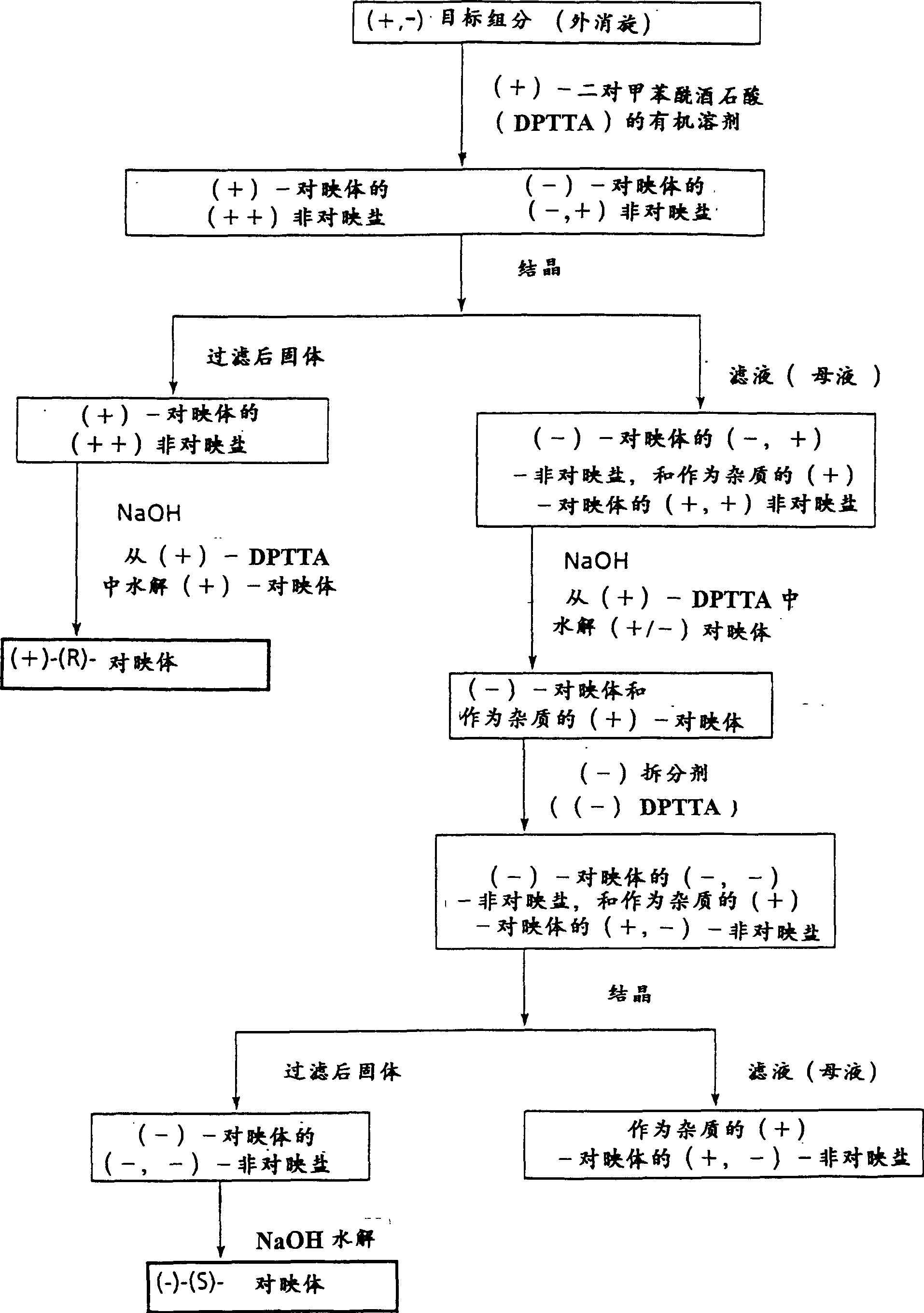
Diastereomer salts of terfenadine
A process and diastereomeric salts useful for the optical resolution of racemic alpha -[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol, 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]- alpha , alpha -dimethylbenzeneacetic acid and lower alkyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]- alpha , alpha -dimethylbenzeneacetates. The process comprises placing into solution a chiral resolving agent, either (+) / (-)-di-paratoluoyltartaric acid or (-) / (+)-mandelic acid, in an amount equimolar to a compound corresponding to the desired enantiomer of the above compound, precipitating the resulting diastereomeric salt between the chiral resolving agent and the target enantiomer and separating the enantiomer.
Owner:MERRELL DOW PHARMA INC
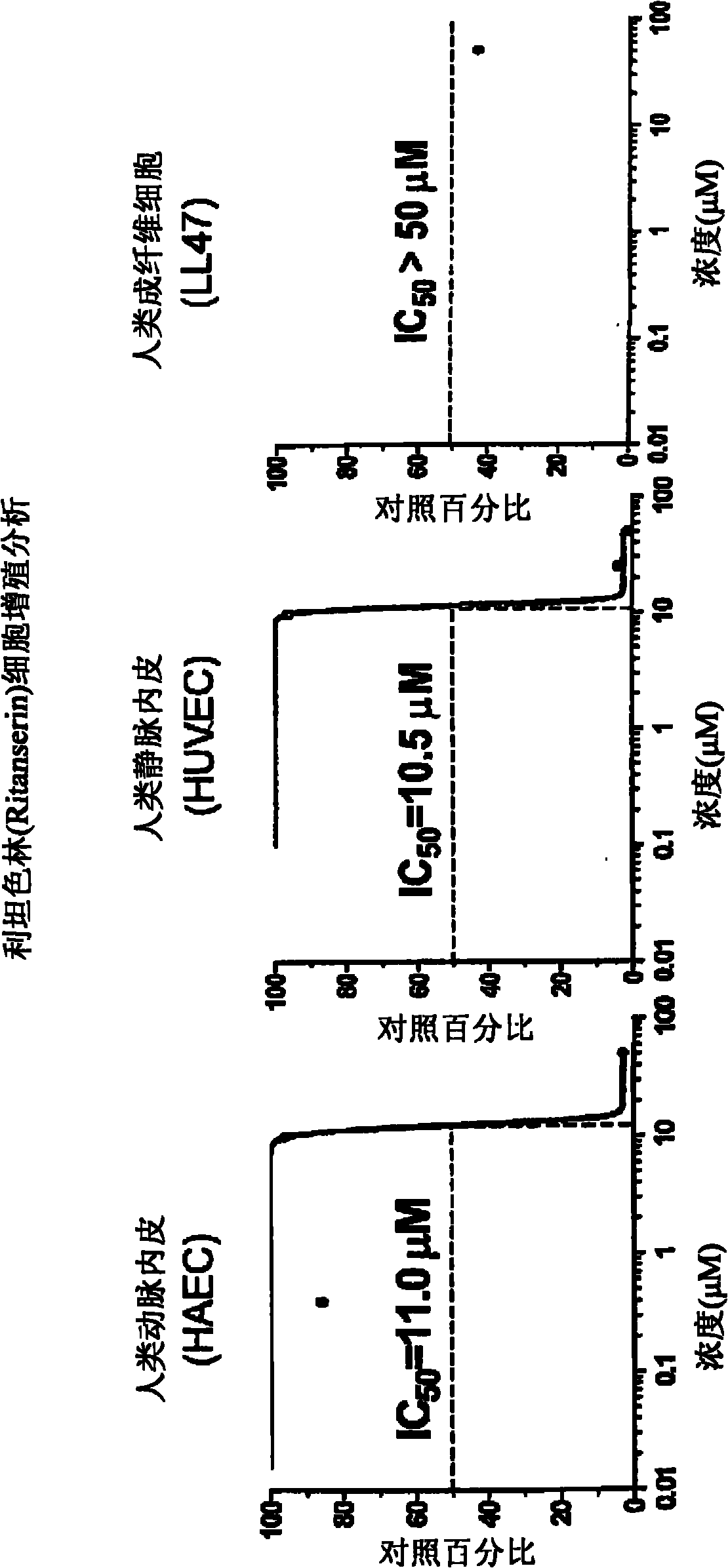
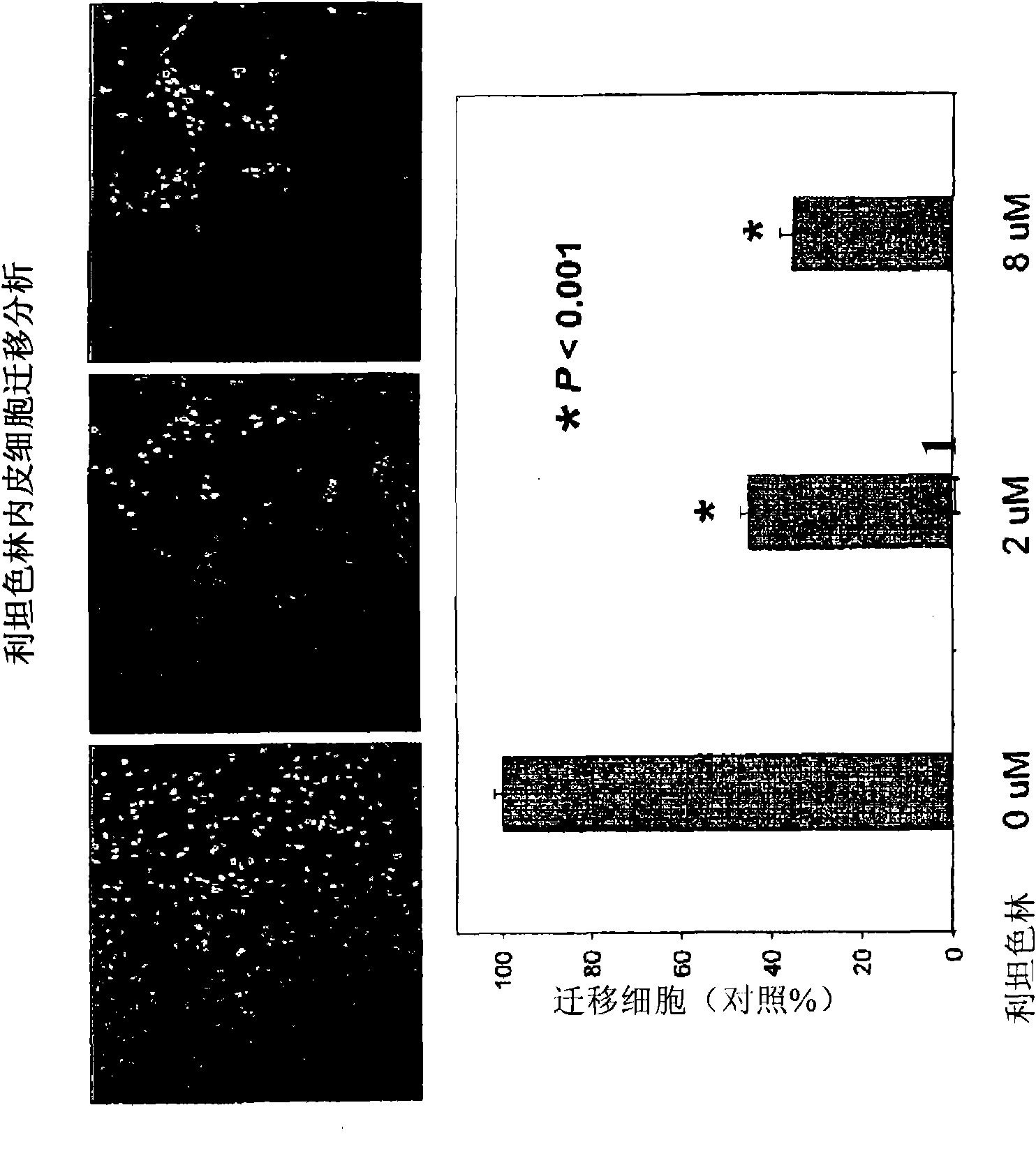
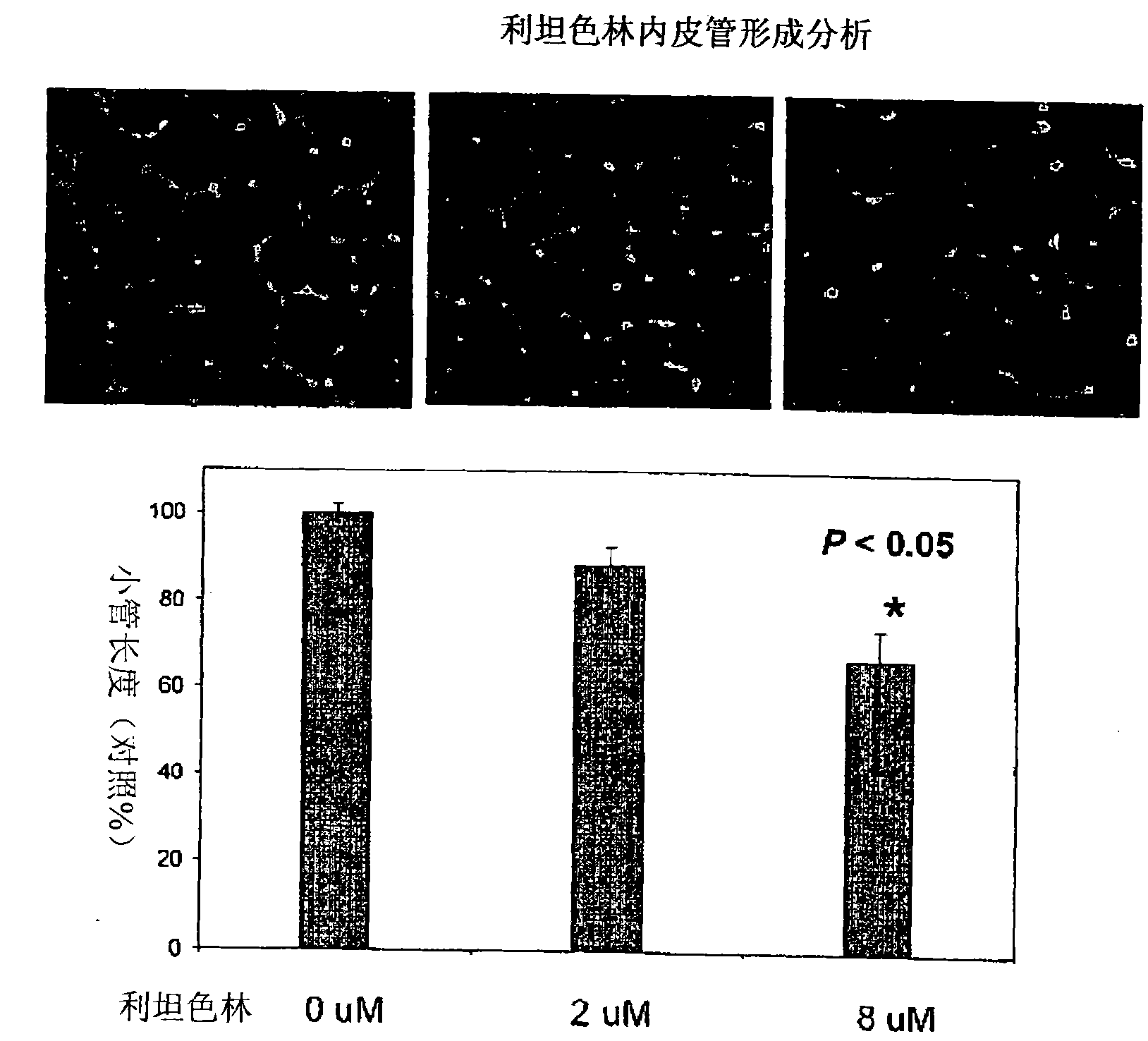
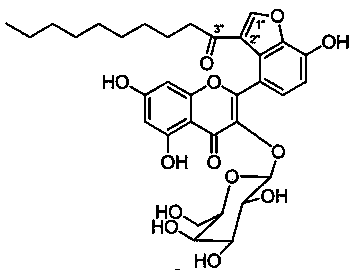
Anti-angiogenic agents and methods of use
Owner:SOUTHERN RES INST & IP
Application of Houttuynoid C in preparation of medicine for treating rheumatoid arthritis
InactiveCN102988378BStrong inhibitory activitySignificant progressOrganic active ingredientsAntipyreticDiclofenac SodiumCurative effect
The invention discloses Houttuynoid C for treating immune-inflammation and the Houttuynoid C is applied to preparing a medicine for treating rheumatoid arthritis. Terfenadine and diclofenac sodium are taken as reference, and the Houttuynoid C is clear in curative effect. The application of Houttuynoid C in preparation of a medicine for treating rheumatoid arthritis is disclosed for the first time, and because the framework type belongs to a novel framework type, the inhibitory activity on the rheumatoid arthritis is unexpectedly high, the possibility that other compounds give enlightenment is avoided, and the Houttuynoid C has outstanding substantial characteristics and has obvious progress in treatment of rheumatoid arthritis.
Owner:何晓涛
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com