Anti-angiogenic agents and methods of use
An anti-angiogenesis and angiogenesis technology, applied in anti-inflammatory agents, cardiovascular system diseases, medical preparations containing active ingredients, etc., can solve the problem of limited number of anti-angiogenesis agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0224] The preparation of such prodrug derivatives is discussed in various literature sources (examples are: Alexander et al., J. Med. Chem., 1988, 31, 318; Aligues-Martin ( Aligas-Martin et al., PCX WO pp / 41531, p. 30). The nitrogen functionality converted in the preparation of these derivatives is one (or more) of the nitrogen atoms in the compounds of the invention.
[0225] Prodrug forms of carboxyl compounds of the present invention include esters (-CO 2 R), wherein the R group corresponds to any alcohol having a pharmaceutically acceptable level of release in vivo by enzymatic or hydrolytic processes. Another prodrug derived from the carboxylic acid form of the present invention may be a quaternary salt structure as described by Bodor et al., Journal of Medicinal Chemistry, 1980, 23, 469
[0226]
[0227] It will of course be understood that the compounds of the present invention relate to all optical isomers and stereoisomers at every possible atom in the molecule....
example 1
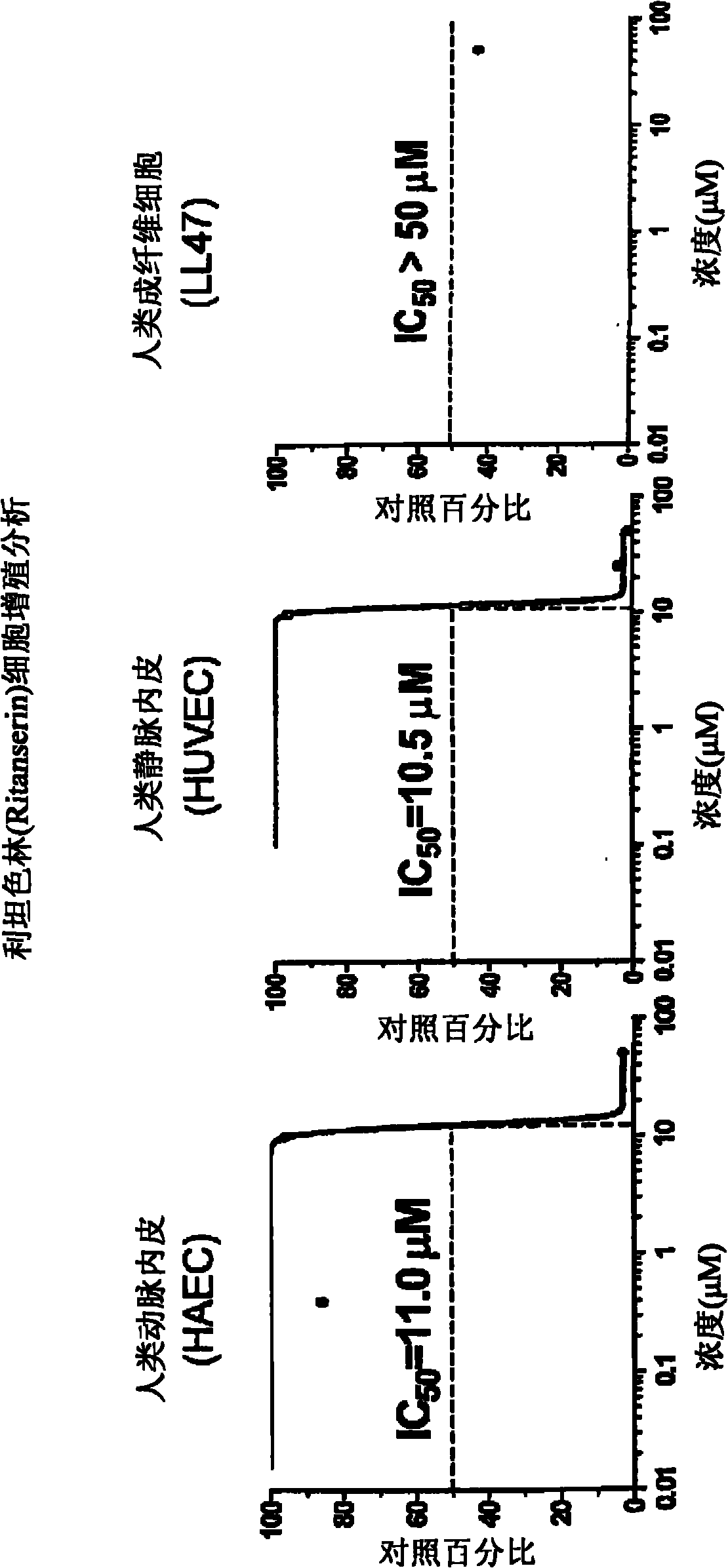
[0232] Cell Proliferation Assay
[0233] Human primary cell lines (arterial endothelial, such as HAEC and HPAEC; venous endothelial HUVEC (from Cambrex BioScience Rockland) and lung fibroblasts LL47 (from American Type Cultures) were cultured according to instructions. American Type Culture Collection) and used it to assess the differential activity of target compounds against human endothelial versus fibroblasts by the CellTiter-Glo luminescent cell viability assay. This assay generates a luminescent signal, It is based on the quantification of ATP content in cell culture. The amount of ATP produced in cell culture reflects the number of viable cells. Therefore, this analysis is often used to assess cell proliferation and the cytotoxic effect of test compounds. Take approximately 5 × 10 3 Cells / well Cells were seeded in growth medium in 96-well plates. After 24 hours, various doses of the compound of interest were added to the cultures, each dose replicated four times. Afte...
example 2
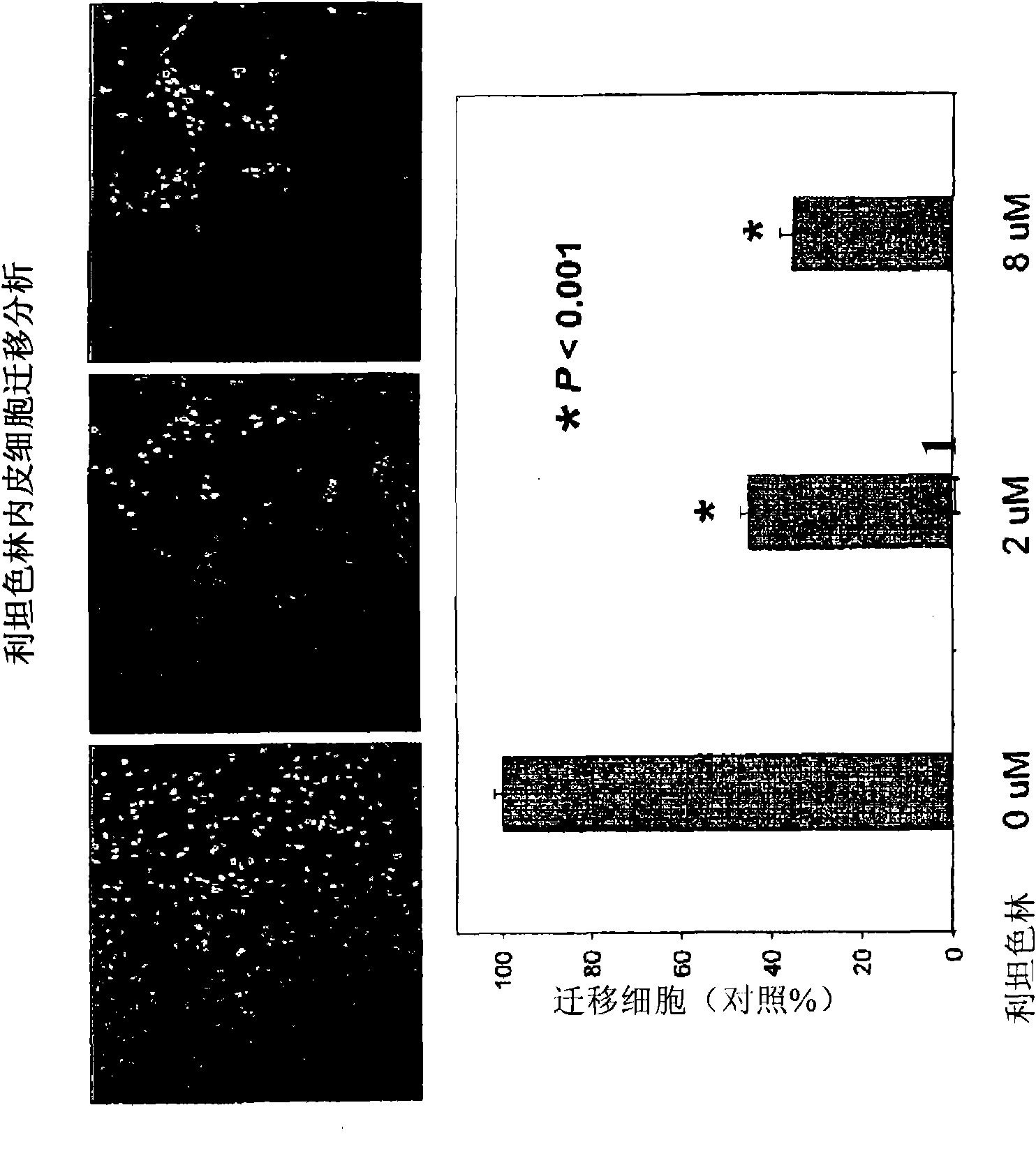
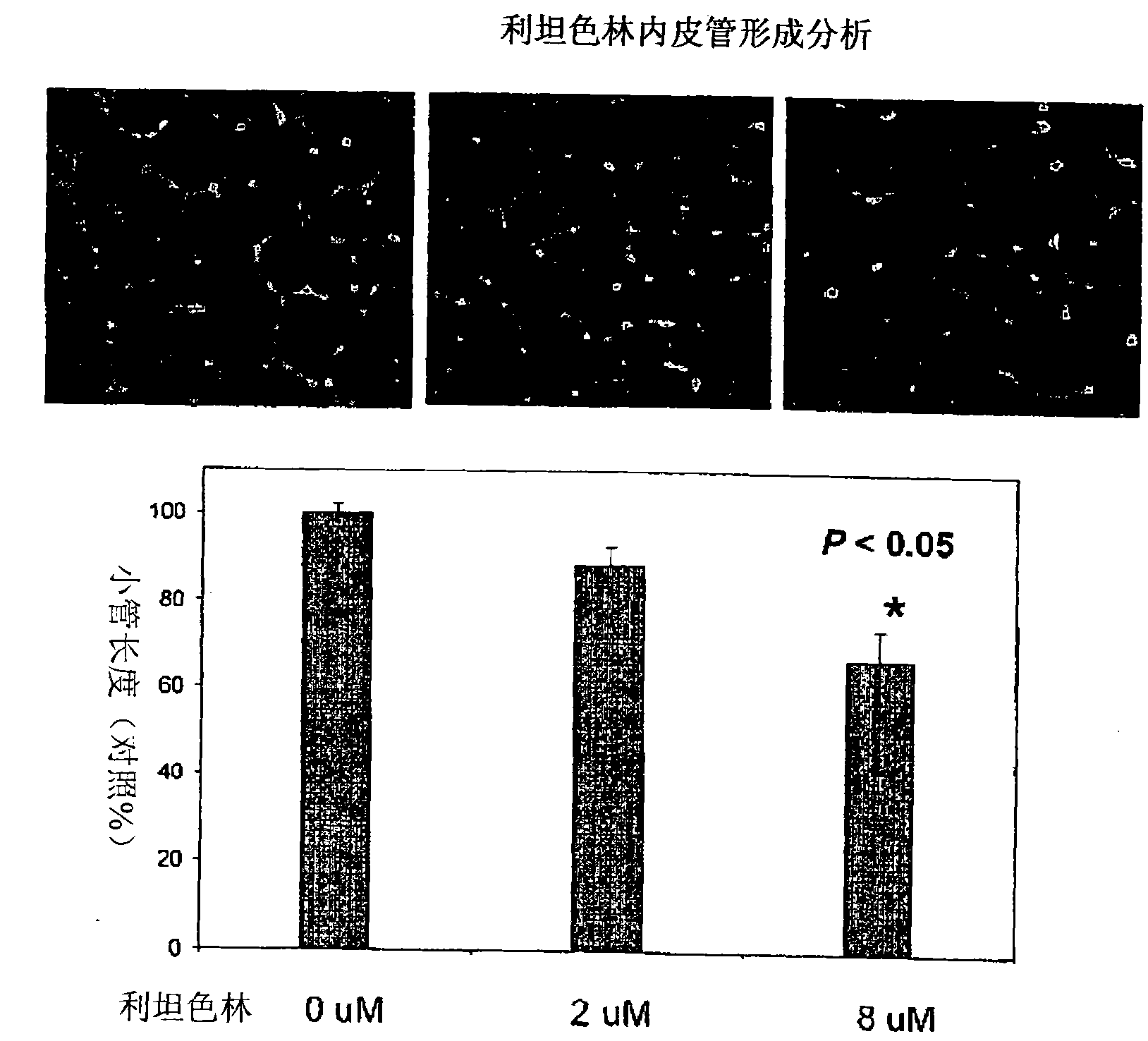
[0235] Endothelial cell migration assay
[0236]Endothelial cell migration is a critical step in the angiogenic process that is decisive for the in situ recruitment response to vessel formation. Endothelial cell migration assays were performed using the permeable membrane chamber filter / insert chamber of the Biocoat Endothelial Cell Migration Angiogenesis System (BD Biosciences), which contains human fibronectin-coated 24-permeable membrane chamber plate with 3-μm pore size inserts. Inserts were incubated with endothelial cell basal medium containing 0.1% bovine serum albumin for 1 hr at 37 °C. Endothelial cells (HUVEC) were starved for 4 to 5 hours with 0.1% bovine serum albumin in endothelial cell basal medium, after which the cells were harvested and subsequently seeded (1×10 5 per well) in the upper chamber of a permeable membrane well plate, where each treatment was performed in 100 μl of 0.1% bovine serum albumin in endothelial cell basal medium. Add complete growth m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com