Novel composition for treating psoriasis
A technology of psoriasis and composition, which is applied in the field of new composition for treating psoriasis, can solve problems such as side effects, and achieve the effect of not easy to relapse, simple preparation process and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
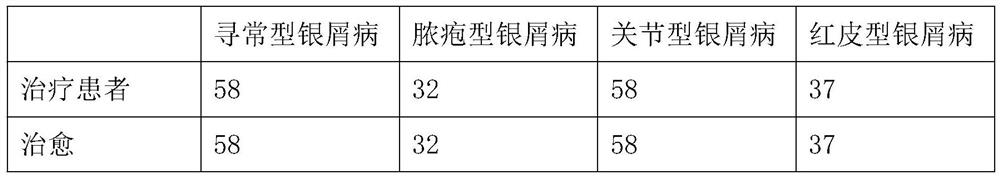
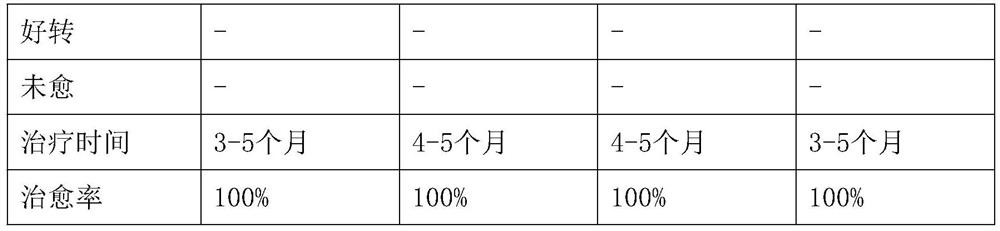
Image
Examples
Embodiment 1
[0042] A novel composition for treating psoriasis, comprising the following components in parts by weight: 0.005 parts of ethyl aryretinoin, 1 part of polypeptide, 0.07 parts of ribose, 15 parts of terfenadine, 50 parts of vitamin E, ammonia 500 parts of peptide peptide, 50 parts of aminophylline, 1 part of chlorpheniramine maleate, 100 parts of vitamin C, 13 parts of vitamin B, 1.5 parts of vitamin B, 10 parts of tripterygium glycosides, 4.58 parts of microcrystalline cellulose, 3.33 parts of pregelatinized starch, 6.67 parts of crospovidone, and 1 part of talcum powder; wherein the pregelatinized starch is 1500 parts, and the crospovidone is PVP K30.
[0043] Described composition is oral capsule, and its preparation method comprises:
[0044] Step a: Take each component according to the weight ratio of the above components, place them in a mixer and mix them uniformly, and use the obtained composition for later use;
[0045] Step b: passing the composition in step a throug...
Embodiment 2
[0049] A novel composition for treating psoriasis, comprising the following components in parts by weight: 0.02 parts of ethyl aryretinoin, 6 parts of polypeptide, 0.27 parts of ribose, 60 parts of terfenadine, 200 parts of vitamin E, ammonia Copeptide 2000 parts, aminophylline 200 parts, chlorpheniramine maleate 10 parts, vitamin C 400 parts, vitamin B112 parts, vitamin B210 parts, tripterygium glycosides 40 parts, microcrystalline cellulose 18.33 parts, pregelatinized 13.33 parts of starch, 26.67 parts of crospovidone, and 4 parts of talcum powder; among them, the pregelatinized starch is 1500 parts, and the crospovidone is PVP K30.
[0050] Described composition is oral tablet, and its preparation method comprises:
[0051] Step a: Take each component according to the weight ratio of the above components, place them in a mixer and mix them uniformly, and use the obtained composition for later use;
[0052] Step b: the composition in step a is placed in a tablet press, and ...
Embodiment 3
[0055] A novel composition for treating psoriasis, comprising the following components in parts by weight: 0.01 part of ethyl aryretinoin, 3.5 parts of polypeptide, 0.15 part of ribose, 35 parts of terfenadine, 130 parts of vitamin E, ammonia Copeptide 1200 parts, aminophylline 130 parts, chlorpheniramine maleate 5 parts, vitamin C 250 parts, vitamin B17 parts, vitamin B26 parts, tripterygium glycosides 25 parts, microcrystalline cellulose 11 parts, pregelatinized 8 parts of starch, 16 parts of crospovidone, and 2.5 parts of talcum powder; among them, the pregelatinized starch is 1500 parts, and the crospovidone is PVP K30.
[0056] Described composition is oral capsule, and its preparation method comprises:
[0057] Step a: Take each component according to the weight ratio of the above components, place them in a mixer and mix them uniformly, and prepare the obtained composition for later use;
[0058] Step b: passing the composition in step a through a 100-mesh sieve to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com