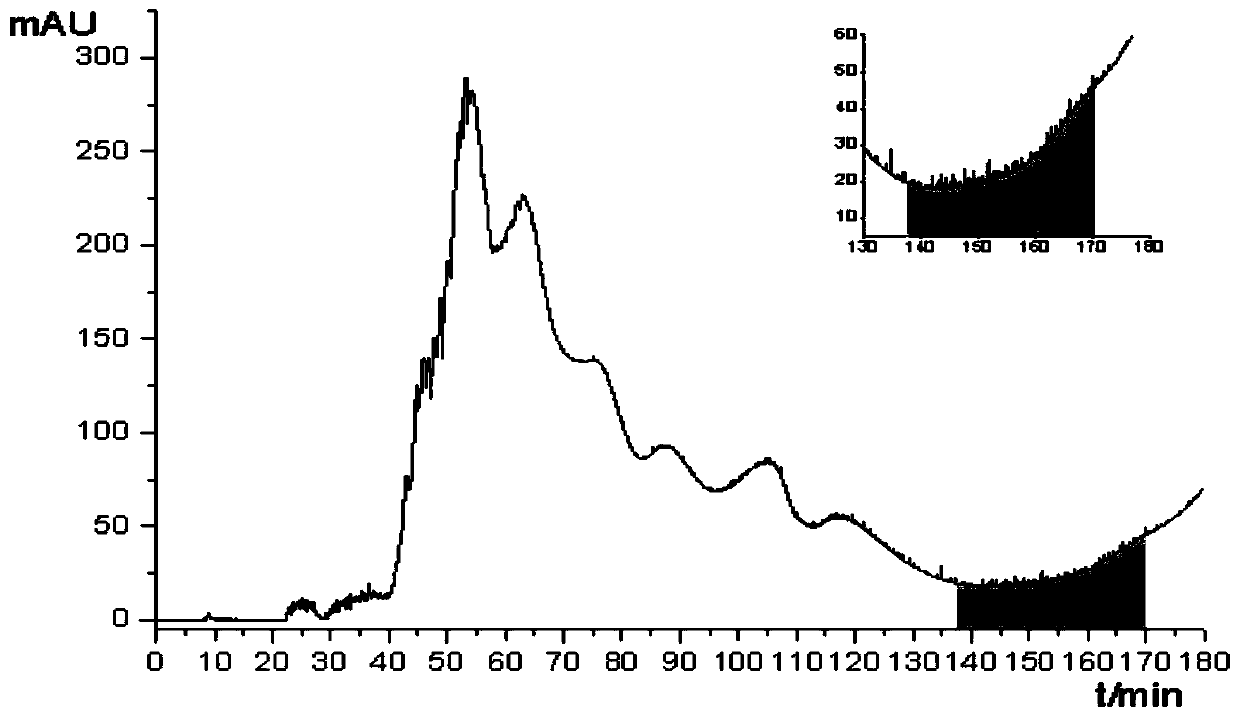
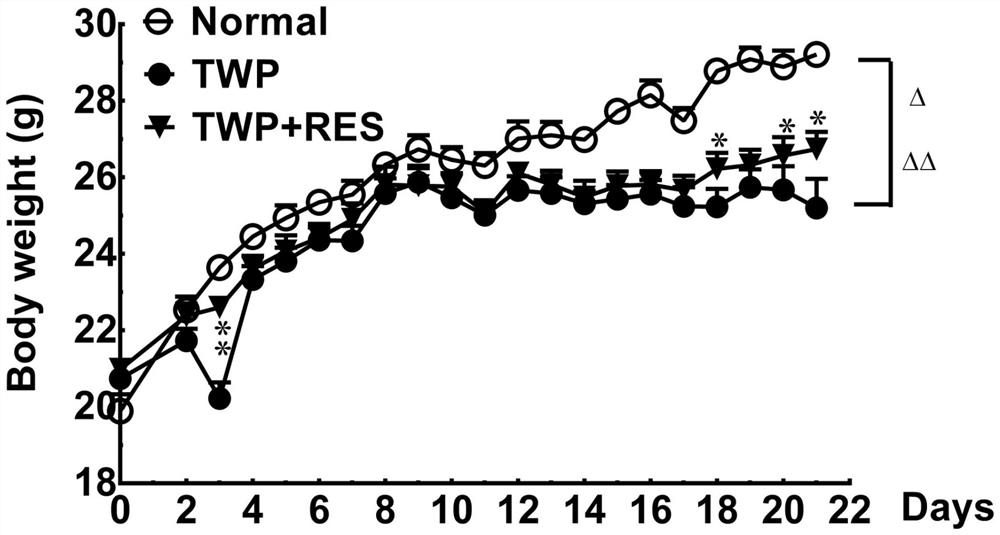
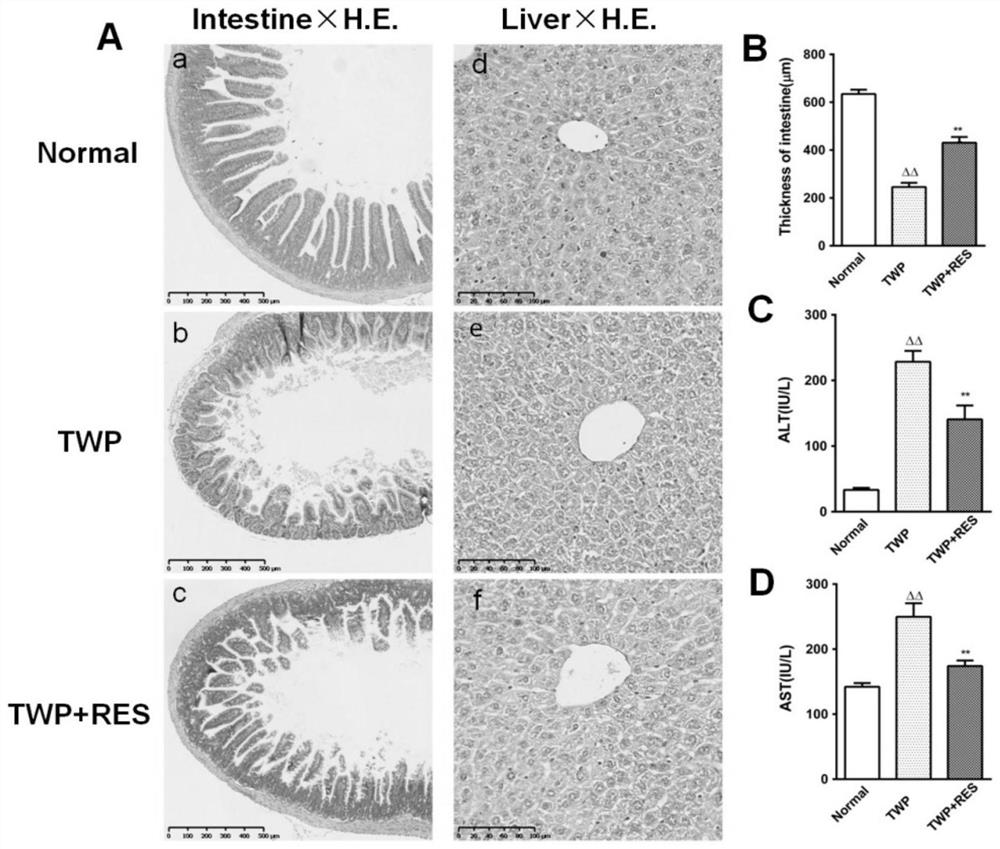
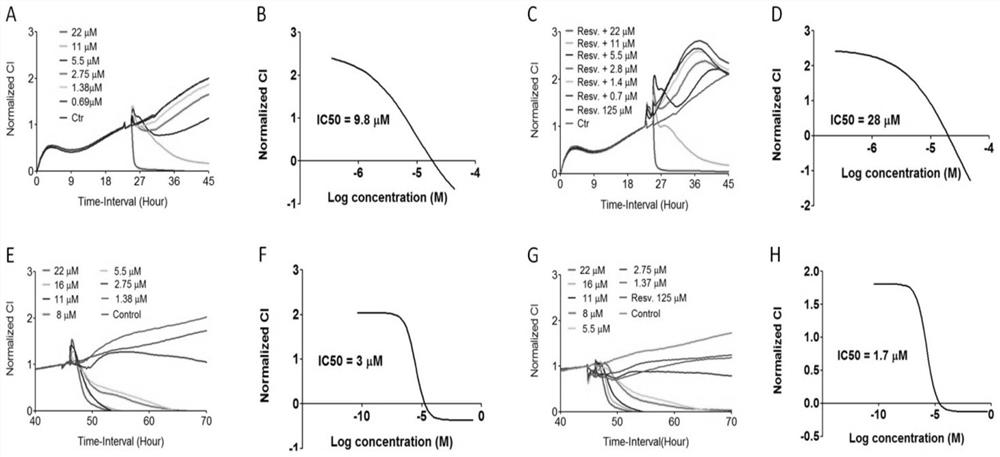
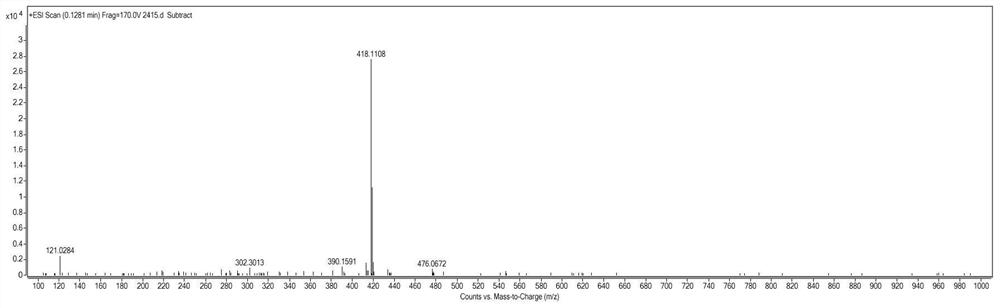
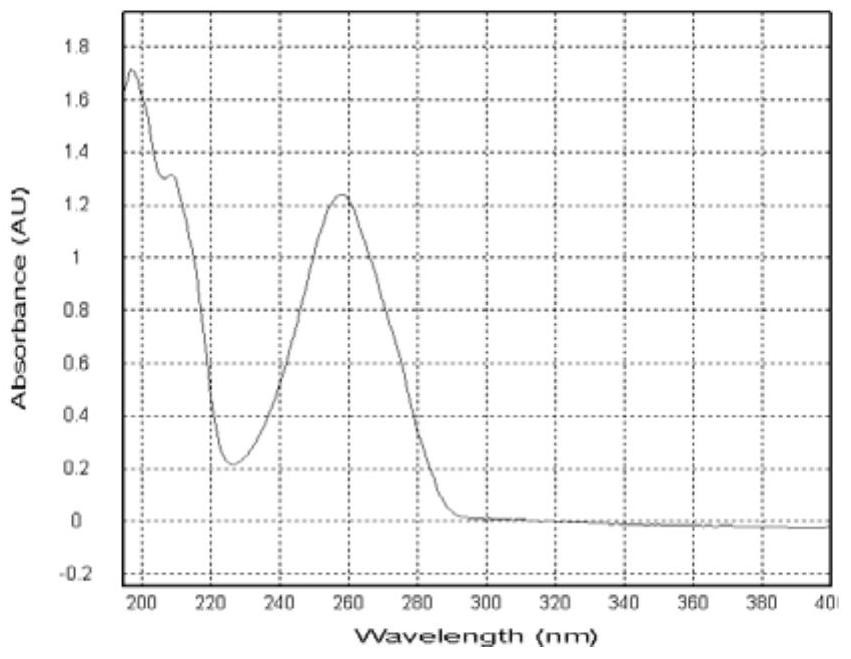
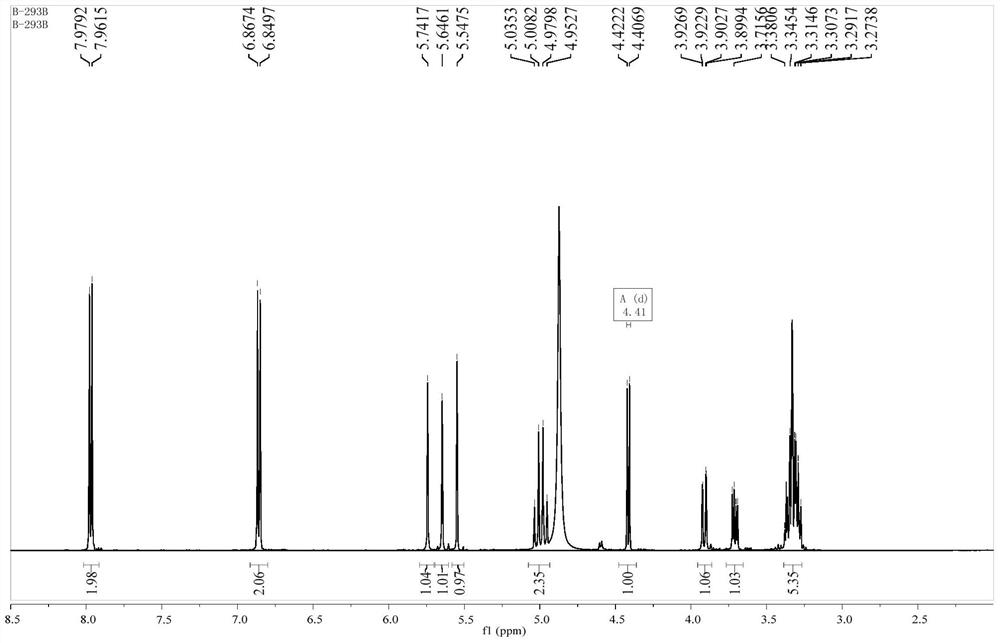
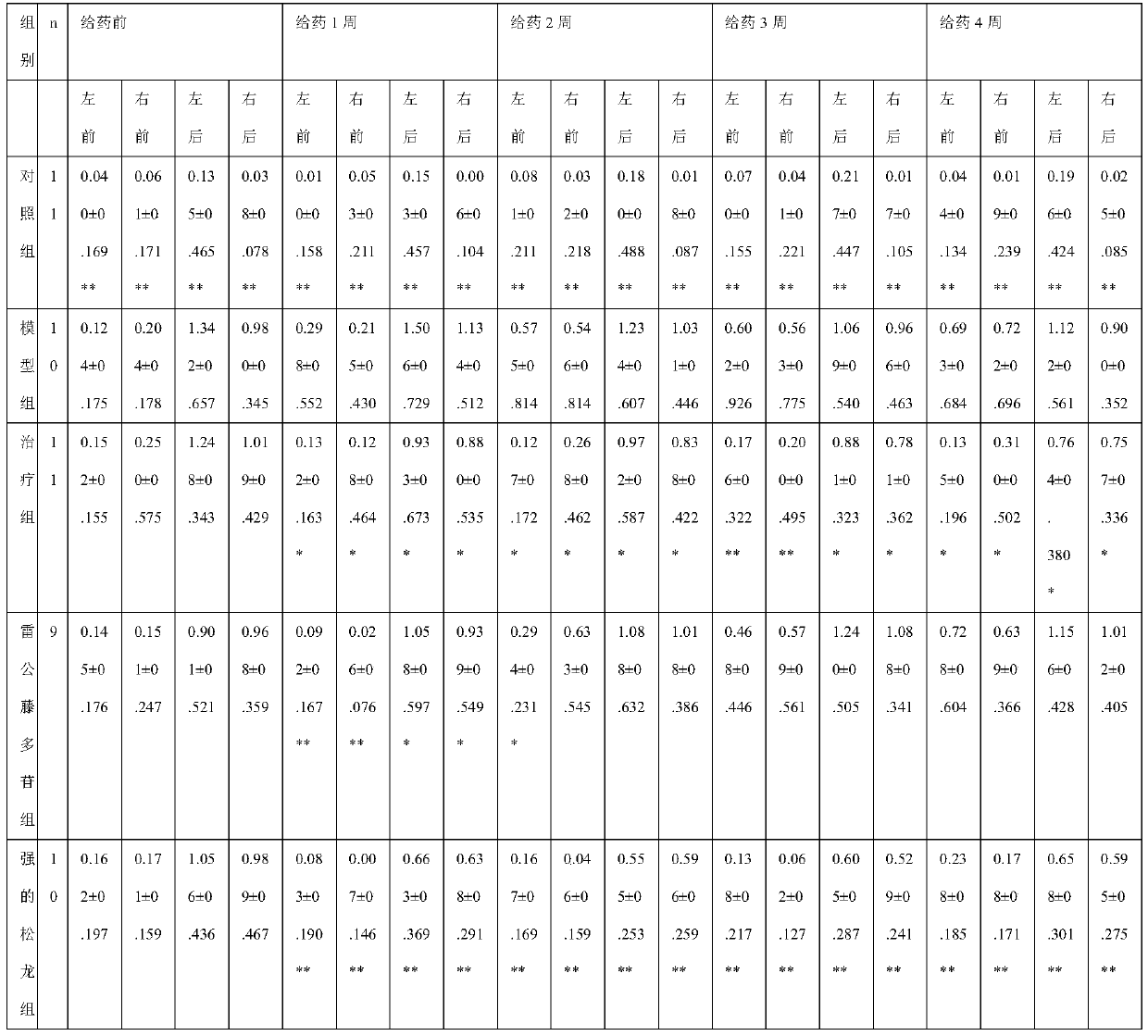
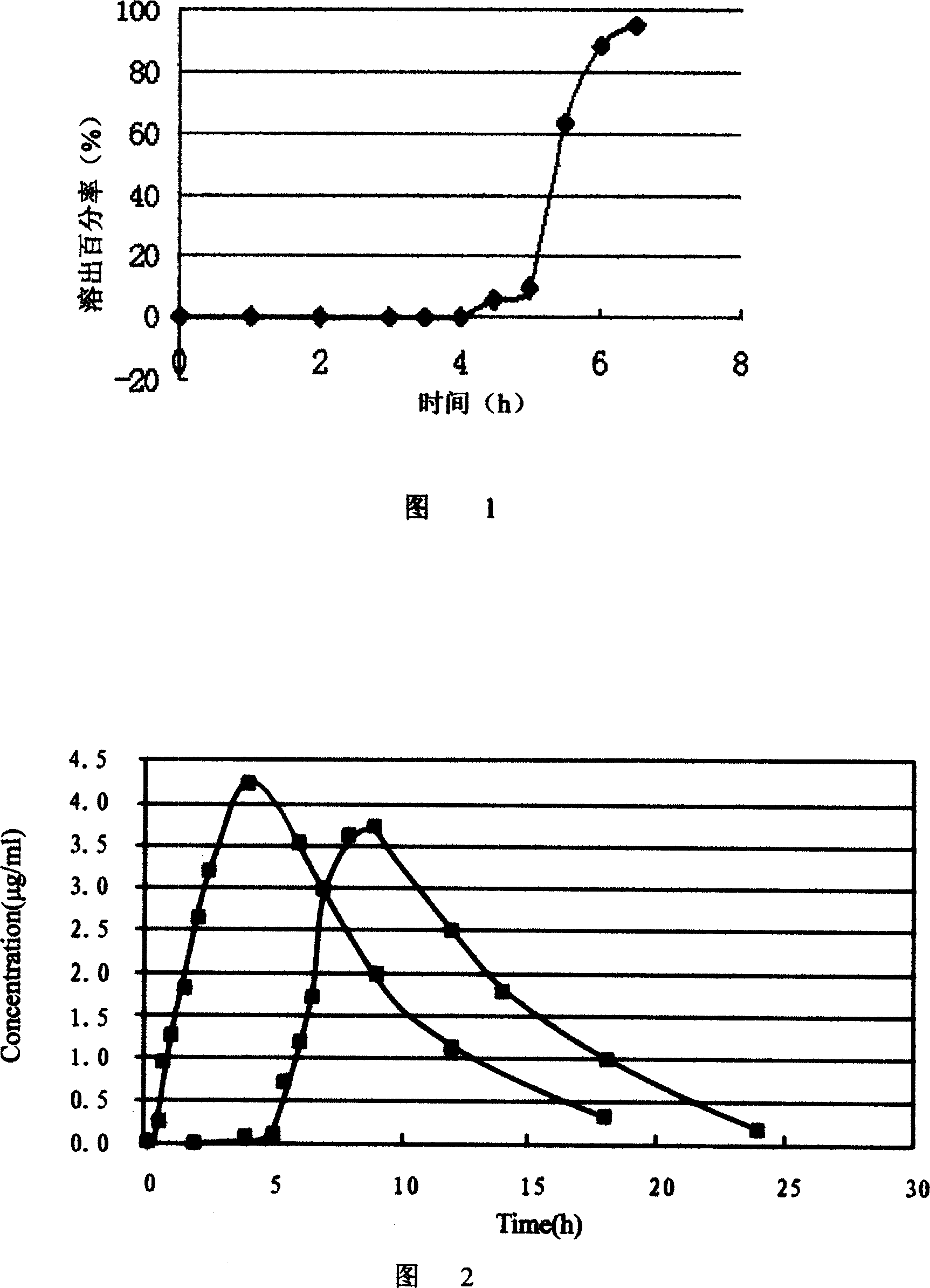
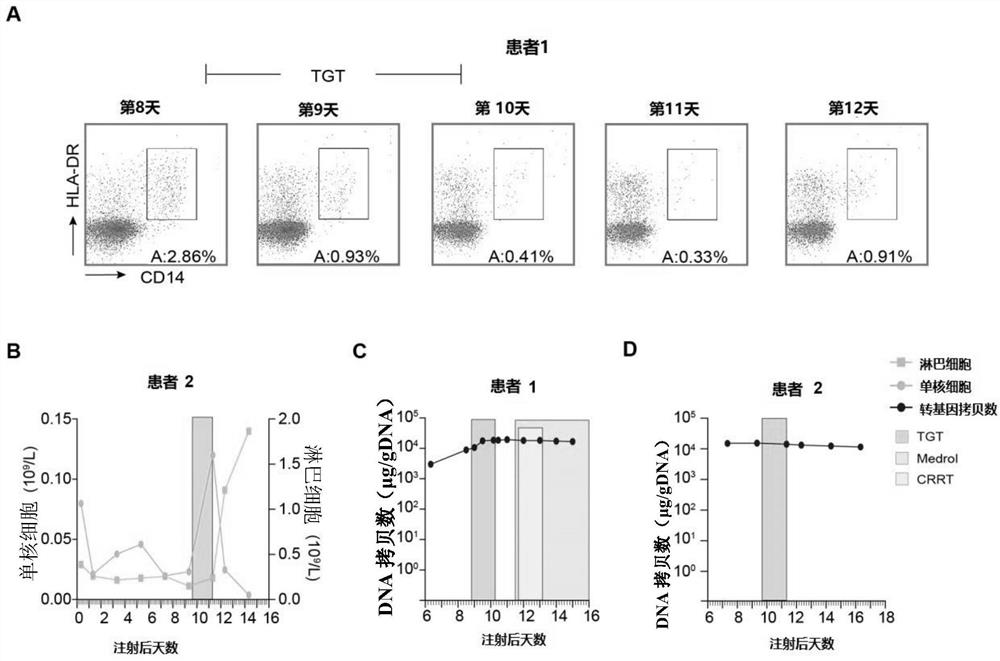
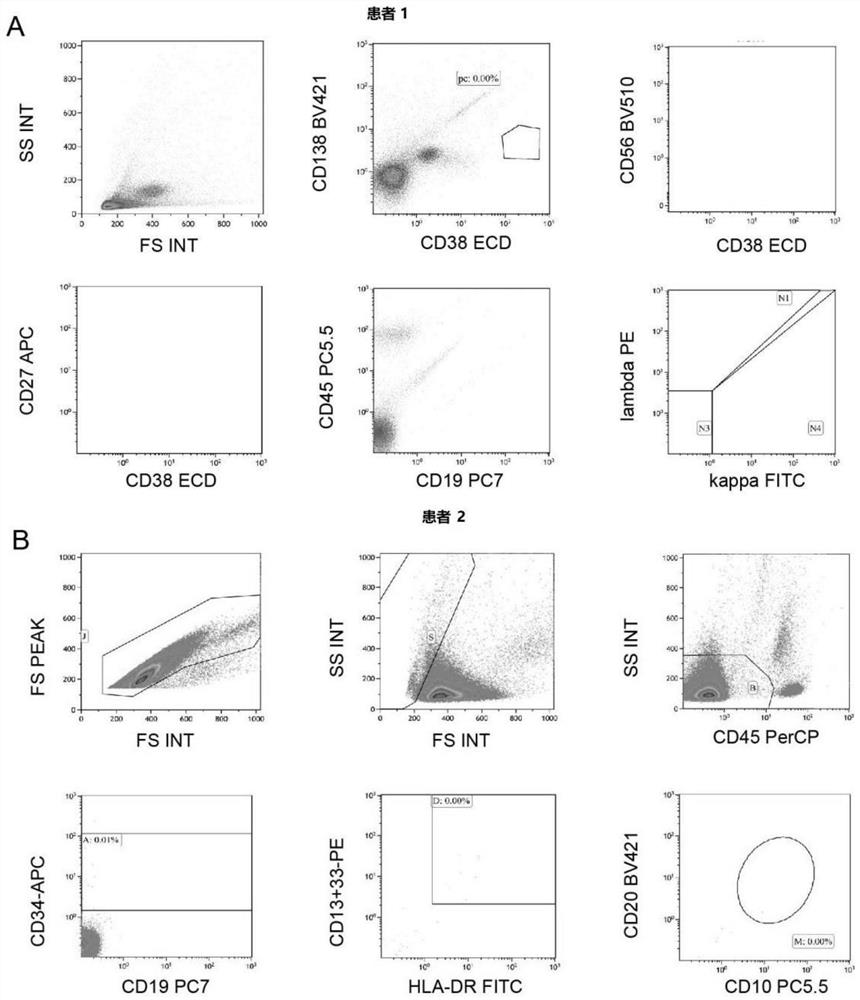
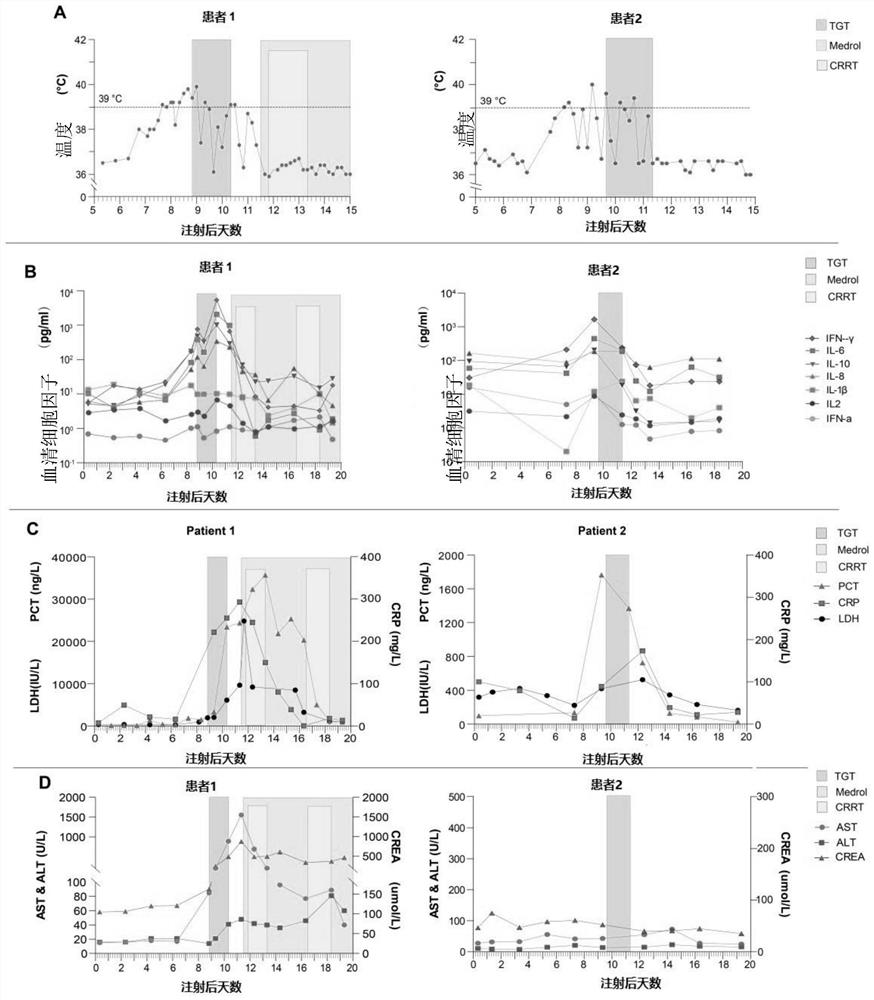
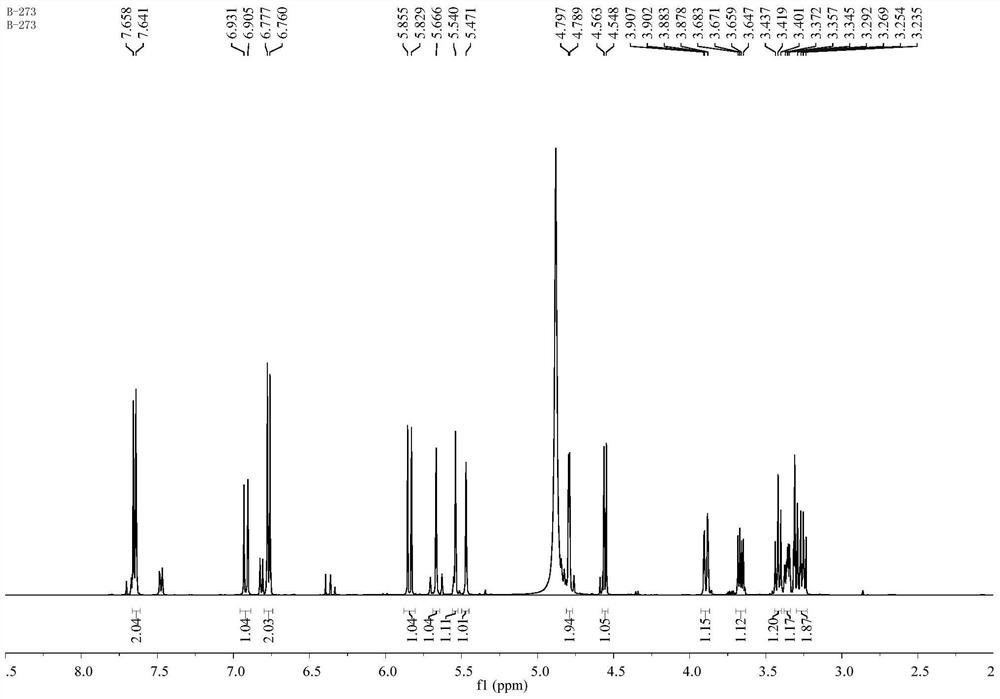
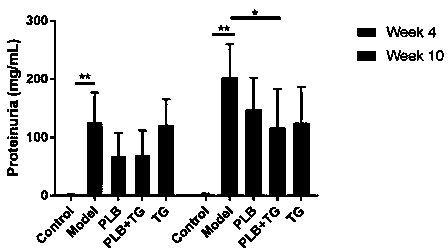
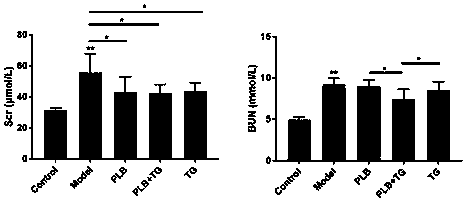
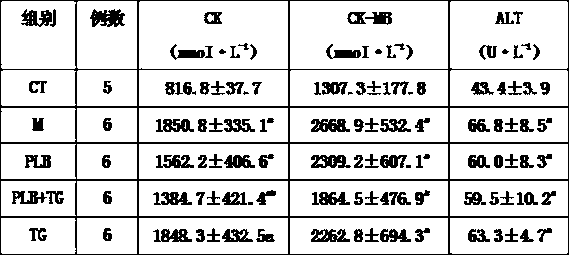
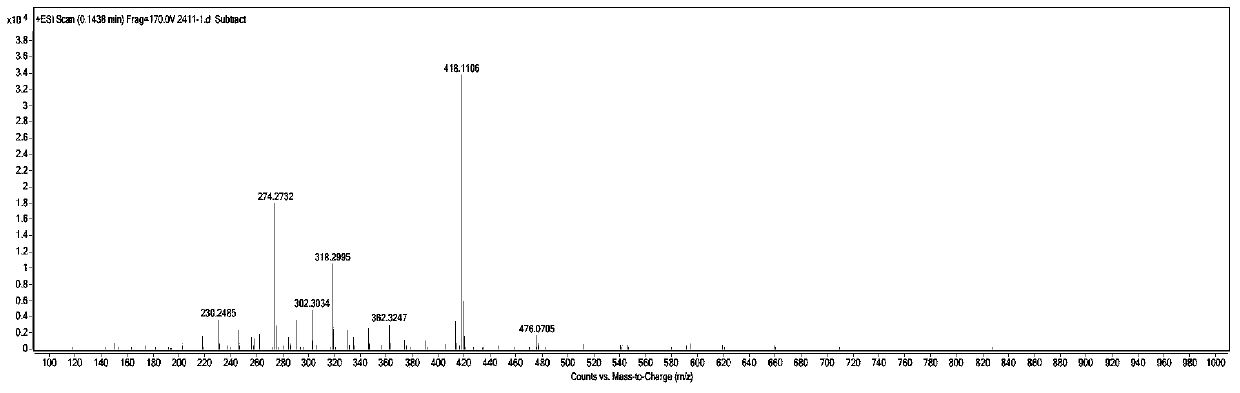
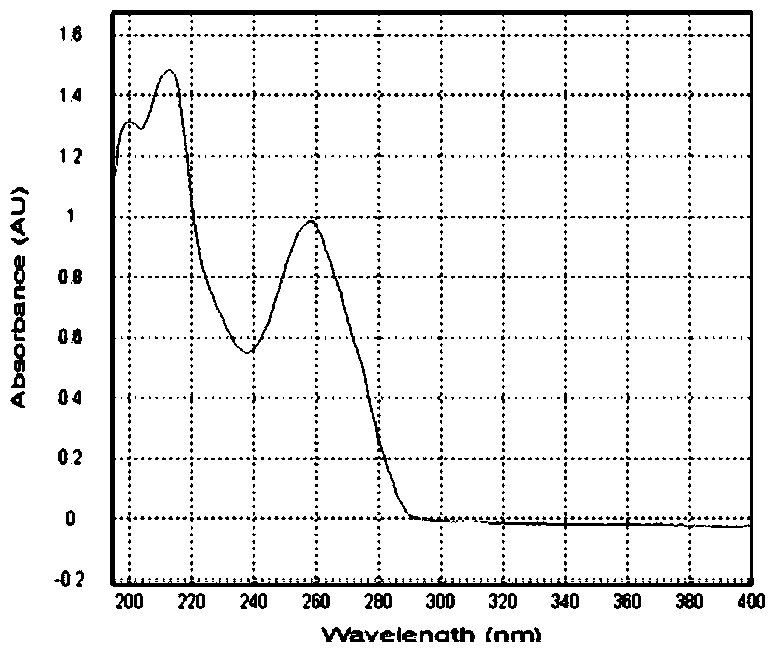
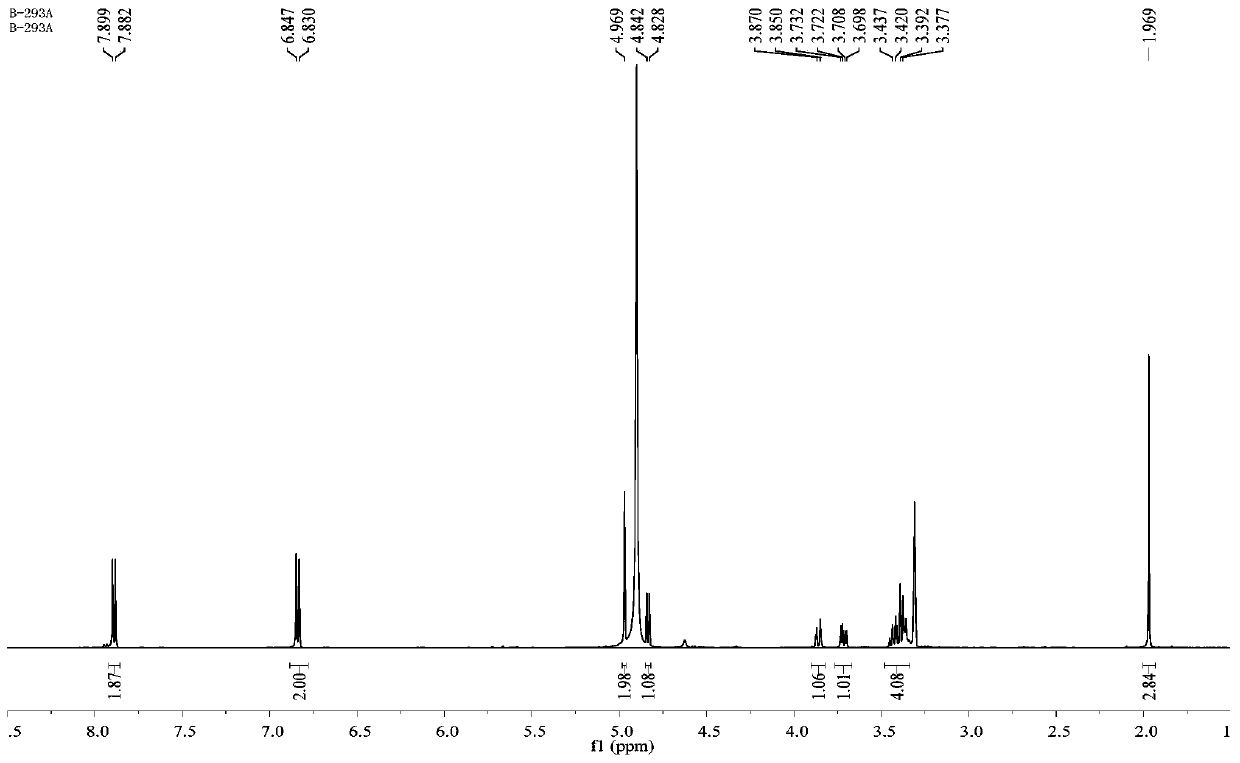
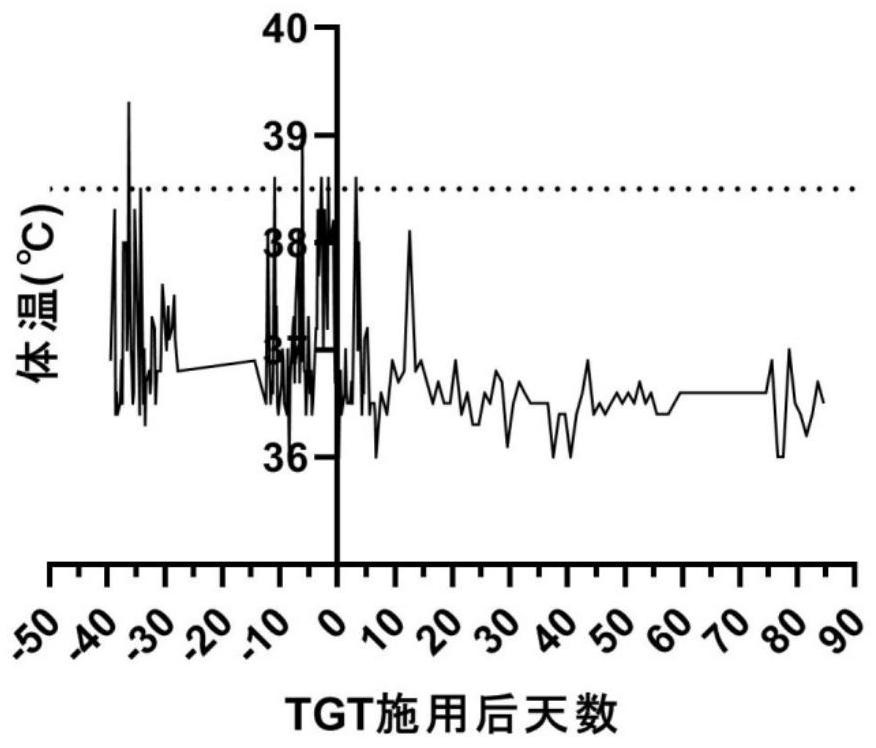
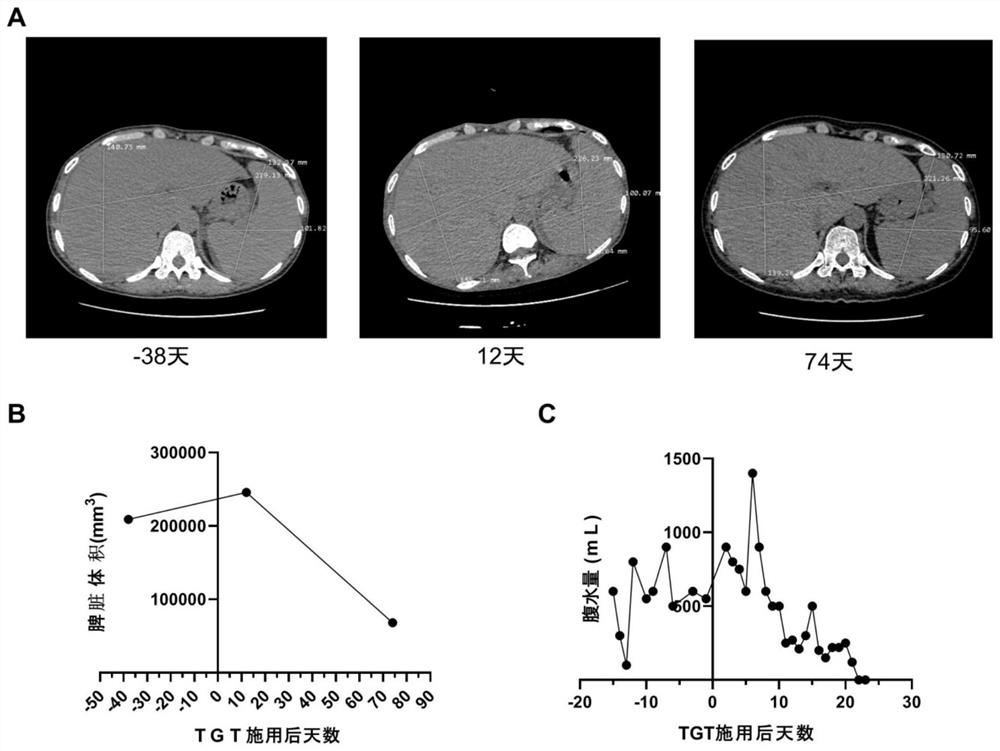
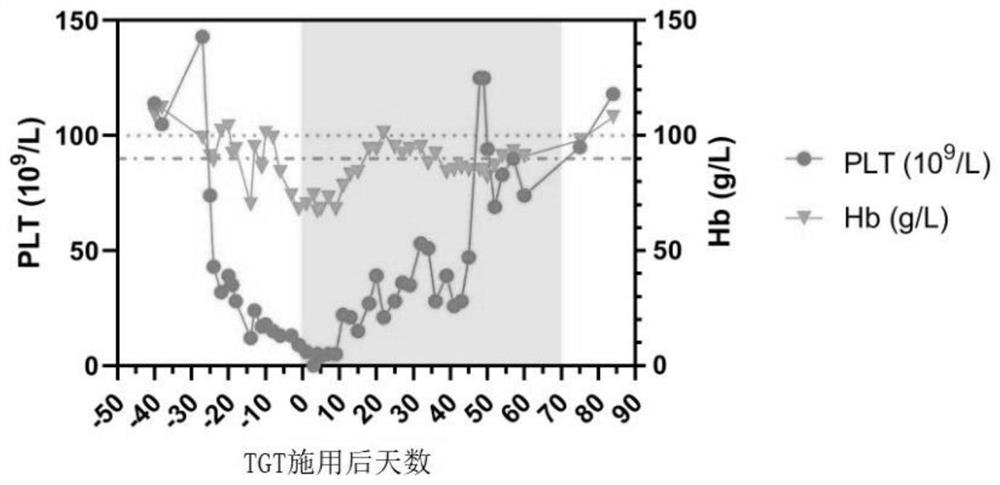
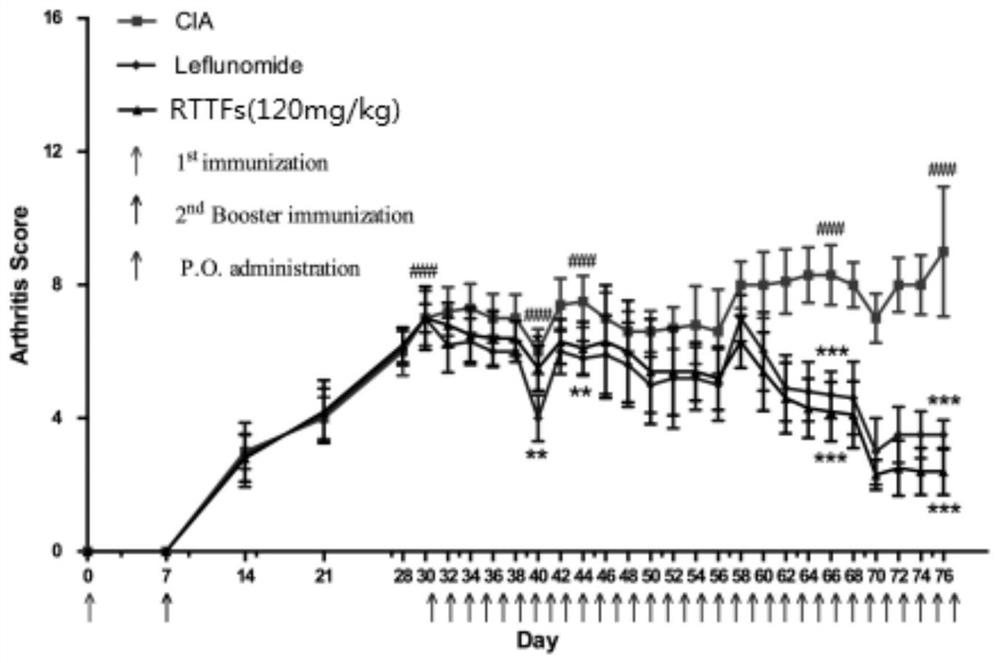
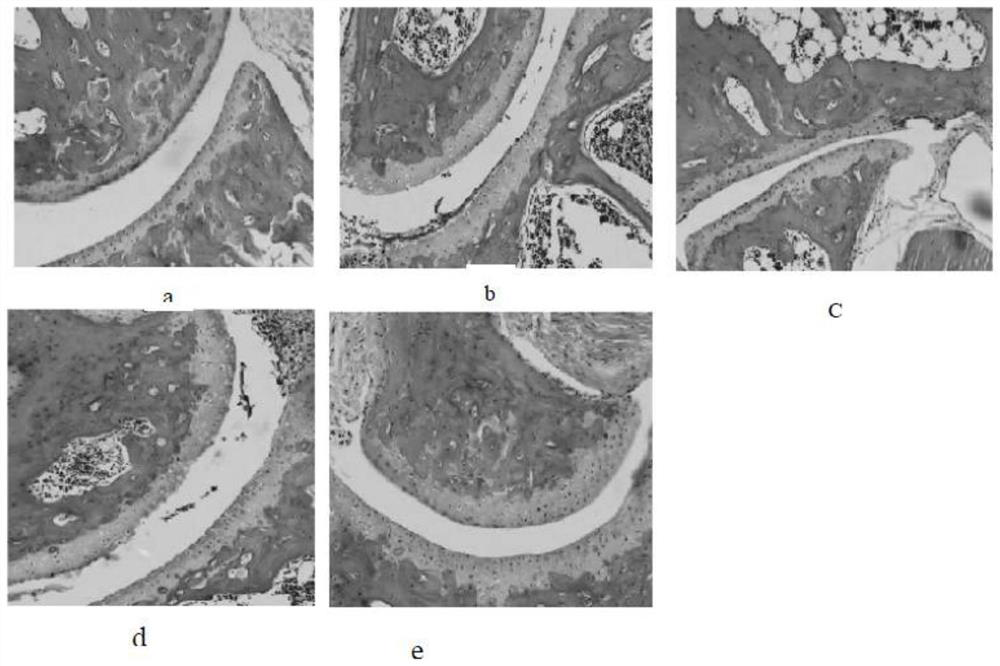
Patents
Literature
40 results about "Multiglycosidorum tripterygii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Multiglycosidorum tripterygii, a new immunosuppressant, supresses coronary arteriosclerosis after heart transplantation. Hachida M(1), Zhang X, Lu H, Hoshi H, Koyanagi H. Author information: (1)Department of Cardiovascular Surgery, The Heart Institute of Japan, Tokyo Women's Medical College.
Tripterygium glycoside-containing micro-emulsified gel transdermal preparation and preparation method thereof
InactiveCN102462723ASimple manufacturing processImprove product qualityAntipyreticAnalgesicsActive agentSurface-active agents
The invention belongs to the technical field of medicinal preparations and relates to a tripterygium glycoside-containing micro-emulsified gel transdermal preparation and a preparation method thereof. The preparation method is characterized in that a gel matrix is added into microemulsion and the mixture is further processed into the tripterygium glycoside-containing micro-emulsified gel transdermal preparation. The tripterygium glycoside-containing micro-emulsified gel transdermal preparation comprises: by mass, 0.1 to 15% of tripterygium glycoside, 15 to 20% of one or more surfactants, 30 to 40% of one or more auxiliary surfactants, 15 to 25% of oil phases, 15 to 25% of water, 0.5 to 2% of one or more transdermal absorption promoters, and 0.5 to 5% of one or more gel base materials. Thetripterygium glycoside-containing micro-emulsified gel transdermal preparation is semitransparent or transparent, has nattier blue opalescence and a pH value of 6 to 10 and can form micro-emulsion having particle sizes of 10 to 100nm, can be processed into pasters so that drug transdermal delivery is realized, has low skin irritation, can be prepared by the simple preparation method, and has stable quality and good drug transdermal absorption promotion performances.
Owner:FUDAN UNIV
Tripterygium glycosides nano-emulsion gel and preparation method thereof
ActiveCN107303263AAppropriate viscosityBroaden applicationOrganic active ingredientsAntipyreticAdditive ingredientGlycoside formation
The invention belongs to the technical field of a pharmaceutical preparation and specifically relates to a tripterygium glycosides nano-emulsion gel, a preparation method thereof and an application thereof. The main ingredients of the tripterygium glycosides nano-emulsion gel disclosed by the invention include raw material tripterygium glycosides, an oil phase, a surfactant, a cosurfactant, a gel matrix and deionized water. The method comprises the following steps: mixing tripterygium glycosides with the oil phase, surfactant and cosurfactant; dropwise adding the deionized water into the mixed solution; magnetically stirring, thereby acquiring the tripterygium glycosides nano-emulsion; and adding the gel matrix, thereby acquiring the tripterygium glycosides nano-emulsion gel. The invention selects an O / W type nano-emulsion as a transdermal drug delivery carrier for preparing a tripterygium glycosides nano-emulsion gel transdermal preparation and provides a novel effective drug preparation for the clinical application of tripterygium glycosides.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Compound Tripterygium wilfordii multi-glycosides formulation for curing rheumatic disease and preparation method thereof
InactiveCN101297841AImprove joint damageReduce congestion and edemaOrganic active ingredientsAntipyreticDiseaseTreatment effect
The invention relates to a compound tripterygium glycoside preparation for treatment for rheumatic diseases and a preparation method thereof. The invention takes tripterygium glycoside and artemether as main drugs and is in combination with pharmaceutical excipients, microcrystalline cellulose, lactose, low-substituted hydroxyproxyl cellulose, tiny powder silica gel and magnesium stearate for preparing tablets. The weight ratio is that: 5g of tripterygium glycoside and 5 to 30g of artemether. The invention proposes to allow the tripterygium glycoside and the artemether to be compatible to realize the synergy of the efficacies, and the compatibility of the tripterygium glycoside and the artemether has higher treatment effects on rheumatic diseases than that of the tripterygium glycoside.
Owner:JIANGXI HERBFINE HI TECH
Method for extracting tripchlorolide from tripterygium wilfordii front segment
The invention discloses a method for extracting tripchlorolide from tripterygium wilfordii front segment extractum. At present, tripchlorolide with little amount is obtained by employing a multi-time chromatography method to perform chromatographic separation on tripterygium glycosides or an extraction product directly extracted from tripterygium wilfordii raw material by ethanol and perform rotating thin layer chromatography, and the method cannot be used to large-scale production at all. The method provided by the invention comprises: utilizing a hydrophilic solvent low alcohol / ketone to perform extraction on the tripterygium wilfordii front segment extractum, separating majority of lipophilic impurities in the front-segment extractum, then extracting with trichloromethane to separate some other non-target products with relatively small solubility in the low alcohol / ketone, then performing chromatographic separation purification, and finally utilizing chloroform to perform recrystallization. The method provided by the invention is simple and practicable in separation and purification technology, is suitable for industrialized large-scale production; and the prepared tripchlorolide is applicable to develop new drugs.
Owner:浙江得恩德制药股份有限公司
Method for reducing organic solvent residue in tripterygium glycoside
The invention relates to a method for reducing an organic solvent residue in tripterygium glycoside. Organic solvents (mainly comprising ethanol and chloroform) are adopted to carry out collection, extraction, chromatography and the like in a production technology of the tripterygium glycoside, and the problem of an unstable index of the organic solvent residue in the tripterygium glycoside is generated at present. The method comprises the following steps: dissolving a tripterygium glycoside semi-finished product by absolute ethyl alcohol, and fully agitating to evenly dissolve; concentrating the obtained tripterygium glycoside ethanol solution, and pumping out at reduced pressure after recovering ethanol, so as to obtain a powdery dry product; and obtaining the tripterygium glycoside product by adopting a method of combining warm water precipitation with low-temperature vacuum drying. By adopting the method, the organic solvent residue in the tripterygium glycoside can be effectively reduced, and the quality is stable.
Owner:浙江得恩德制药股份有限公司
A kind of method for extracting tripterygium chloractone from tripterygium wilfordii extract
Owner:浙江得恩德制药股份有限公司
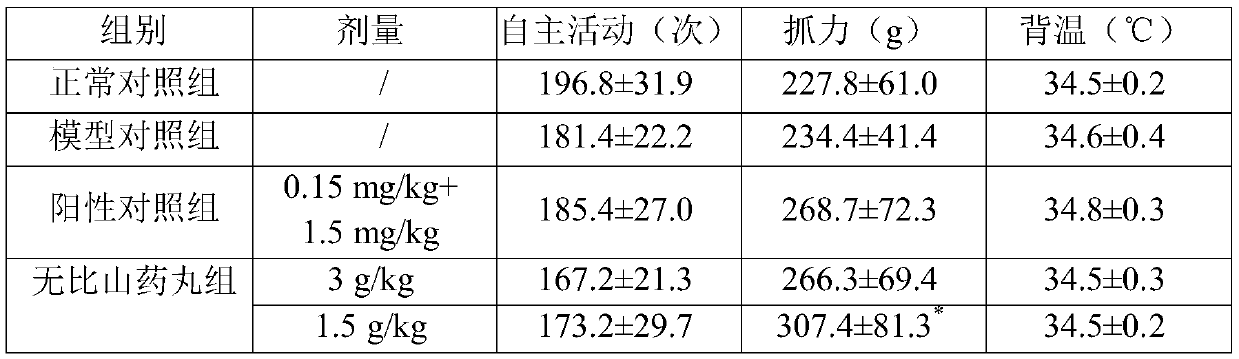
Application of Wubi Shanyao pills to preparation of POI (premature ovarian insufficiency) treatment drug
InactiveCN111514222AIncrease the number ofImprove gripHeavy metal active ingredientsPill deliveryRehmanniae RadixMultiglycosidorum tripterygii
The invention discloses application of Wubi Shanyao pills to preparation of a POI (premature ovarian insufficiency) treatment drug. The Wubi Shanyao pills are prepared from rhizoma dioscoreae, herba cistanche, fructus schizandrae, semen cuscutae, cortex eucommiae, radix achyranthis bidentatae, rhizoma alismatis, radix rehmanniae praeparata, fructus corni, poria cocos, radix morindae officinalis and halloysitum rubrum. A female ICR mouse is subjected to intragastric administration by adopting tripterygium glycosides for 10 weeks to induce POI, molding is carried out, then the model mouse is continuously subjected to intragastric administration by adopting the Wubi Shanyao pills for 12 weeks, it is found that compared with a model control group, the Wubi Shanyao pill administration group hasthe advantages that the holding power of the model mouse can be obviously increased, the average estrous cycle days are shortened, the number of granular cell layers and the number of preantral follicles and sinus follicles are increased, the serum LH level is reduced, the Wubi Shanyao pills have a remarkable improvement effect on the POI mouse, so that the POI treatment or improvement effect isachieved, and good news is brought to the POI patient.
Owner:HANGZHOU HUQINGYUTANG PHARM CO LTD
Tripterygium wilfordii medicinal material and method for determining wilforlidea in tripterygium wilfordii polyglycosides tablet
ActiveCN110687224AReduce dosageHigh recovery rateComponent separationChromatographic separationLiquid liquid partition
The invention provides a tripterygium wilfordii medicinal material and a method for determining wilforlidea in a tripterygium wilfordii polyglycosides tablet. The method comprises the following stepsof taking an ethanol solution of 0.1 mg.mL<-1> of wilforlidea as a reference substance solution; acquiring a corresponding volume ratio range by establishing and optimizing normal hexane, ethyl acetate, ethanol and water of a solvent system so that a distribution coefficient K of the wilforlidea in the solvent system is 0.5-2; optimizing and controlling a rotating speed of a liquid-liquid distribution chromatographic separation column and a flowing speed of a mobile phase, and detecting the tripterygium wilfordii medicinal material and the method for determining the wilforlidea in the tripterygium wilfordii polyglycosides tablet by combining high performance liquid chromatography. The method is simple in operation process, high in specificity and good in reproducibility, separation cost isgreatly reduced, a wilforlidea chromatographic separation degree is high, a product recovery rate is high too, a sample dosage is small, stability is good, precision is high, qualitative and quantitative accuracy is high too, and quality of the tripterygium wilfordii medicinal material and the tripterygium wilfordii polyglycosides tablet can be effectively evaluated.
Owner:ZHEJIANG UNIV OF TECH
A kind of tripterygium glycosides and tripterygium monomer synergistic and attenuated toxicity compatibility method
ActiveCN107468742BImprove IC50Reduce apoptosisHydroxy compound active ingredientsImmunological disordersSide effectLeukemia
The invention relates to the field of traditional Chinese medicine, and discloses a method for synergizing and attenuating toxicity of tripterygium glycosides and tripterygnee monomers, in which tripterygium polyglycosides or tripterygnee monomers are compatible with resveratrol as a medicine application. The technology of the present invention uses the tripterygium wilfordii main component tripterygium glycosides or tripterygium and resveratrol compatibility to play a synergistic and detoxifying effect. Resveratrol can increase the IC50 of tripteryne on intestinal epithelial cells, and reduce the IC50 of tripteryne on basophilic leukemia cells; it can reduce the apoptosis of intestinal epithelial cells induced by tripteryne; and further found that Veratrol-related effects work by activating Sirt protein. The invention can greatly expand the use of tripterygium wilfordii, tripterygium glycosides and tripterygium wilfordii, reduce the side effects of clinical medication, and reduce pain for clinical patients.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
New polyglycosides of tripterygium wilfordii with low toxicity, its preparation method and application
ActiveCN106860500BLow toxicityReduce pathological damageAntipyreticDigestive systemTherapeutic effectNephrotic syndrome
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Novel composition for treating psoriasis
InactiveCN112641923ALow priceSimple preparation processPeptide/protein ingredientsCarbohydrate active ingredientsInflammatory factorsVitamin C
The invention discloses a novel composition for treating psoriasis. The composition comprises the following components of, in parts by weight, 0.005-0.02 parts of arotinoid ethylester, 1-6 parts of polypeptide, 0.07-0.27 parts of ribose, 15-60 parts of terfenadine, 50-200 parts of vitamin E, 500-2000 parts of aminopeptide, 50-200 parts of aminophylline, 1-10 parts of chlorpheniramine maleate, 100-400 parts of vitamin C, 3-12 parts, 1.5-10 parts of vitamin B2, 10-40 parts of tripterygium glycosides, 4.58-18.33 parts of microcrystalline cellulose, 3.33-13.33 parts of pregelatinized starch, 6.67-26.67 parts of polyvinylpolypyrrolidone and 1-4 parts of talcum powder. The invention provides a method for treating psoriasis by combining traditional Chinese medicines and western medicines. Based on gene regulation and transfer termination of inflammatory factors, cellular immunity and humoral immunity are enhanced at the same time, and the purpose of treating both symptoms and root causes is finally achieved.
Owner:烟台心舒医药科技有限公司
Compound hypoglycemic preparation containing liraglutide and preparation method thereof
InactiveCN106310237ARestore metabolic functionRestore secretory functionPeptide/protein ingredientsMetabolism disorderDuloxetineSide effect
The invention discloses a compound hypoglycemic preparation containing liraglutide and a preparation method thereof. The compound hypoglycemic preparation comprises the following components: liraglutide, Sphallerocarpus Racills polysaccharides, tetrandrine, saccharicterpenin, Spriulina polysacchrides, mixed kinases, potassium magnesium aspartate, fenofibrate, calcium dobesilate, vascular endothelial growth factor, duloxetine, monosialoteterahexosyl ganglioside, tripterygium glycosides, pentoxifylline, and edaravone. The compound hypoglycemic preparation of the invention has a hypoglycemic effect by rectifying metabolic disorder, restoring renal metabolic function, restoring insulin secretion function, promoting regeneration of islet cells, and repairing damaged islet cells; and can resorb and utilize the blood excessive sugar while restoring renal metabolic function. The compound hypoglycemic preparation of the invention is used for eliminating diabetes and the diabetic complications of eyes, kidneys, heart, nerves and limbs, and has the characteristics of distinct therapeutic effect and stable low side effects.
Owner:卢连伟
Compound preparation for reducing hepatotoxicity of tripterygium glycoside tablets, and preparation method of compound preparation
InactiveCN111297814AReduce liver toxicityGood curative effectOrganic active ingredientsAntipyreticEfficacyBiology
The invention discloses a compound preparation for reducing hepatotoxicity of tripterygium glycoside tablets, and a preparation method of the compound preparation. The compound preparation comprises silibin meglumine tablets and the tripterygium glycoside tablets. The compound preparation has the efficacies of protecting the liver for detoxification, tonifying the kidney and strengthening the spleen; the dosage of tripterygium glycosides can be obviously increased; a better curative effect is achieved; and meanwhile, the hepatotoxicity of the tripterygium glycosides is obviously reduced. In addition, according to the compound preparation, the fast release of silibin meglumine and the long-acting stable release of tripterygium glycosides can be realized, and the treatment effect is furtherimproved. In addition, the preparation method provided by the invention has the advantages that the operation is simple; the cost is low; the preparation method is suitable for industrial production;and the application prospects are good.
Owner:HUNAN QIANJIN XIELI PHARMA CO LTD
Preparation of 2r-cardiospermin-5-p-hydroxybenzoate and its application in the preparation of drugs for treating rheumatoid arthritis
ActiveCN106974921BHighlight substantive featuresOrganic active ingredientsSugar derivativesPharmacologyChinese herbology
The invention relates to chemical structure and a preparation method of a compound 2R-cardiospermin-5-p-hydroxybenzoate as well as an application of the compound in preparing medicines for treating rheumatoid arthritis. The 2R-cardiospermin-5-p-hydroxybenzoate, as a novel compound purified and prepared from a conventional traditional Chinese medicine, namely Sorbaria sorbifolia (L.) A. Brown, by the inventor, can be used for obviously inhibiting PEG2 and NO levels in serum of a mouse with adjuvant arthritis, and the compound is close to tripterygium glycosides at an equivalent dosage in activity; therefore, the compound can be used for preparing the medicines for treating and resisting the rheumatoid arthritis. The chemical structure and the preparation method of the compound as well as anti-RA (rheumatoid arthritis) activity of the compound are disclosed for the first time, therefore outstanding substantive features are guaranteed.
Owner:BINZHOU MEDICAL COLLEGE
Traditional Chinese medicine composition and preparation for treating rheumatoid arthritis and preparing method and application thereof
InactiveCN110464773AGood treatment effectSignificant effectAntipyreticAnalgesicsCurative effectTherapeutic effect
The invention discloses a traditional Chinese medicine composition and preparation for treating rheumatoid arthritis and a preparing method and application thereof. The traditional Chinese medicine composition is prepared from the following raw materials in parts by weight: 80-100 parts of helleborus thibetanus, 20-30 parts of schisandra chinensis, 20-30 parts of ganoderma lucidum, 15-30 parts ofradix aucklandiae, 25-50 parts of vervain, 20-30 parts of radix paeoniae rubra, 30-40 parts of nduoh nyaatv buerng, 50-80 parts of a traditional Chinese medicine named 'Sanheliu' according to the Chinese pronunciation, 20-40 parts of polygonum multiflorum, and 30-60 parts of camphor root. The composition has good therapeutic effect on rheumatoid arthritis, and has the curative effect superior to that of tripterygium glycoside.
Owner:江阴持一堂医药科技有限公司
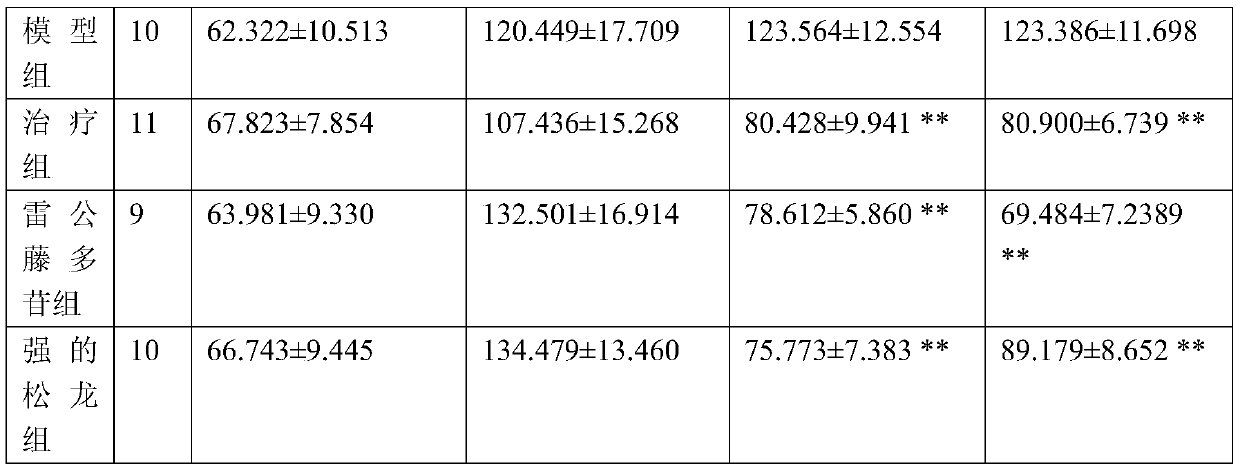
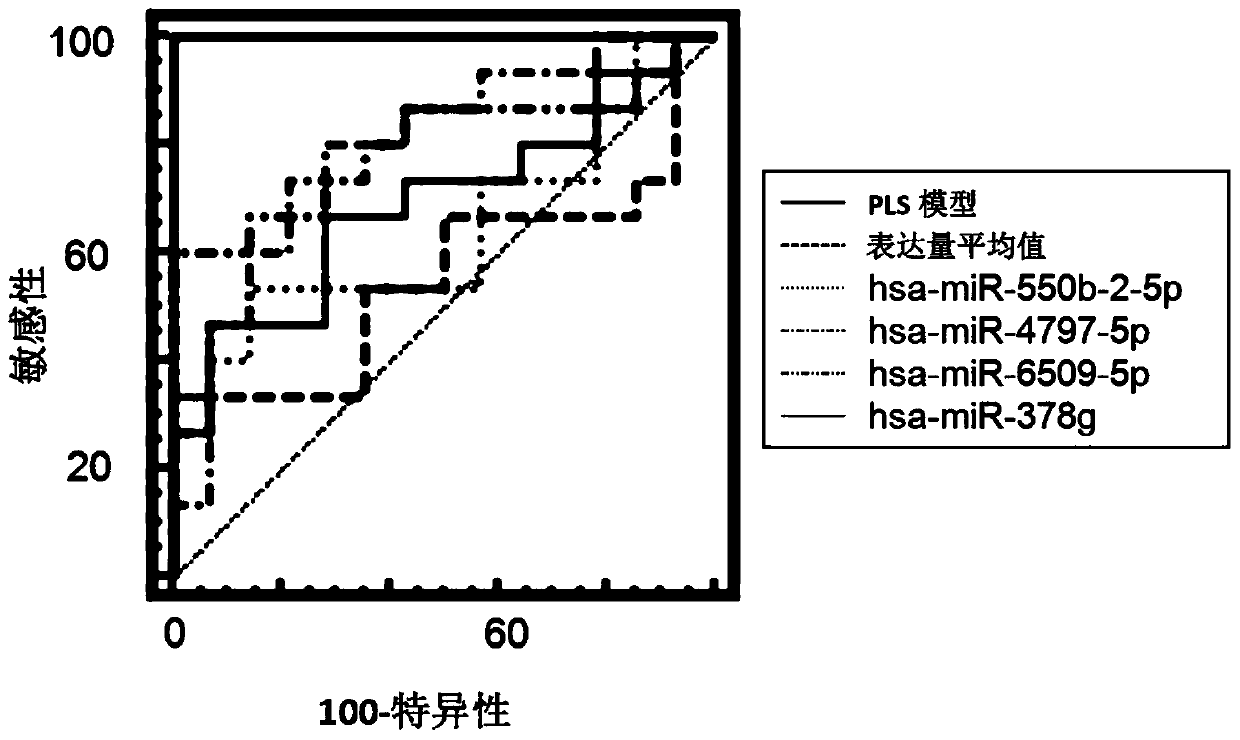
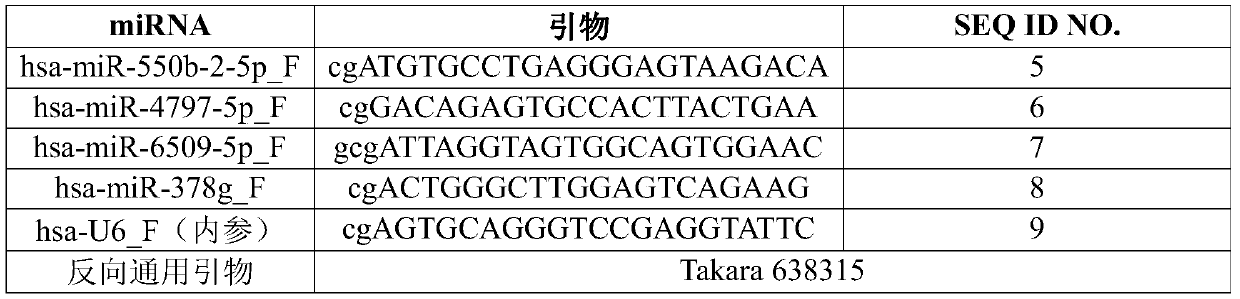
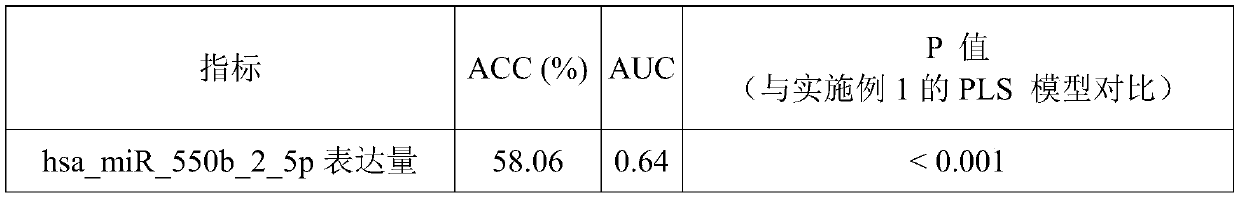
A system for determining the individual effectiveness of tripterygium glycosides in the treatment of rheumatoid arthritis by the expression of multiple miRNAs
The invention provides a system for determining the individual effectiveness of treating rheumatoid arthritis by tripterygium glycosides tablets through the expression amount of various miRNAs (microRibonucleic Acid). The various miRNAs comprise hsa-miR-550b-2-5p, hsa-miR-4797-5p, hsa-miR-6509-5p and hsa-miR-378g. The invention further provides a molecular marker for determining the individual effectiveness of treating the rheumatoid arthritis by the tripterygium glycosides tablets, application of the molecular marker to preparation of a kit for determining the individual effectiveness of treating the rheumatoid arthritis by the tripterygium glycosides tablets, and application of a reagent for detecting the expression amount of the molecular marker to the preparation of the kit for determining the individual effectiveness of treating the rheumatoid arthritis by the tripterygium glycosides tablets. The invention provides a high-specificity and high-sensitivity molecular marker and a curative effect predication model for individual diagnosis and treatment of the rheumatoid arthritis.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Preparation of sutherlandin-5-cis-p-coumarate and its application in the preparation of drugs for treating rheumatoid arthritis
ActiveCN106974923BHighlight substantive featuresOrganic active ingredientsSugar derivativesPharmacologyMultiglycosidorum tripterygii
The invention relates to a chemical structure and preparing method of the compound sutherlandin-5-cis-p-coumarate and application of the compound to preparation of drugs for treating rheumatoid arthritis. Sutherlandin-5-cis-p-coumarate is a novel compound prepared from the traditional Chinese medicine Sorbaria sorbifolia (L.) A. Brown. through purification by the applicant of the invention. The compound can remarkably suppress the level of PEG2 and NO in serum of adjuvant arthritis rats. The activity of the compound is close to that of tripterygium glycosides of the same dose, and the compound can be used for preparing drugs for treating rheumatoid arthritis. The chemical structure, preparing method and anti-RA activity of the compound are disclosed for the first time, thus having prominent and substantive features.
Owner:BINZHOU MEDICAL COLLEGE
Preparation of sutherlandin-5-p-hydroxybenzoate and its application in the preparation of drugs for treating rheumatoid arthritis
ActiveCN106977561BHighlight substantive featuresSugar derivativesAntipyreticPharmaceutical SubstancesHydroxybenzoate
The present invention relates to the chemical structure and preparation method of a compound Sutherlandin-5-p-hydroxybenzoate, and the application of the compound in the preparation of medicines for treating rheumatoid arthritis. Sutherlandin‑5‑p‑hydroxybenzoate is a new compound purified and prepared by the applicant of the present invention from the traditional Chinese medicine Sorbaria sorbifolia (L.) A. Brown. It can significantly inhibit the PEG in the serum of rats with adjuvant arthritis 2 and NO level, and its activity is stronger than that of tripterygium glycosides in the same dose, and can be used to prepare medicines for treating rheumatoid arthritis. The chemical structure, preparation method and anti-RA activity of the compound are disclosed for the first time, so it has outstanding substantive features.
Owner:BINZHOU MEDICAL COLLEGE
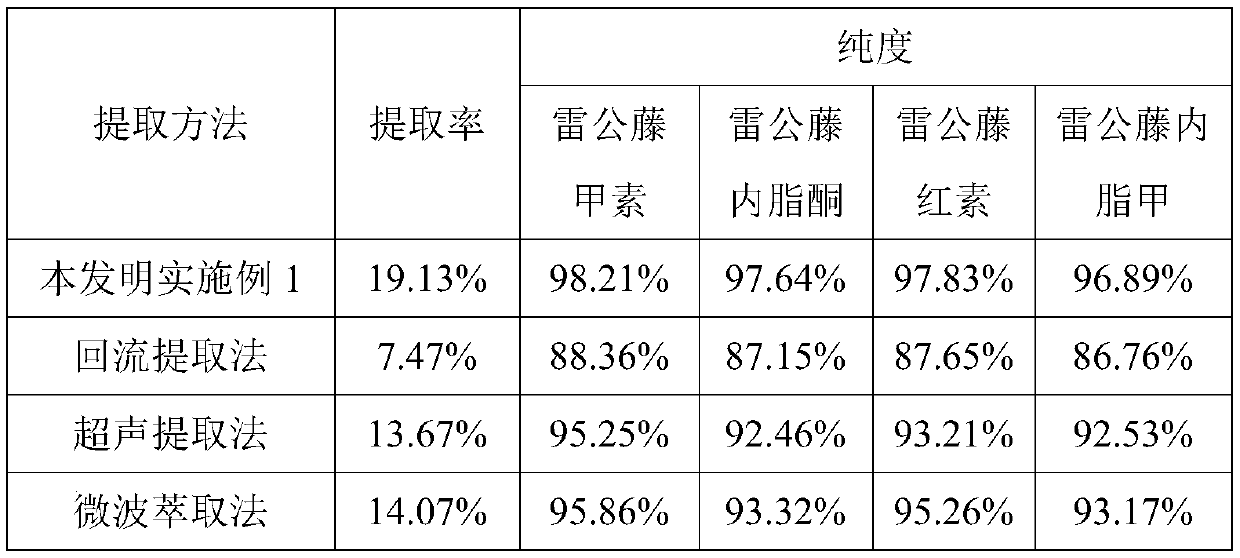
Extraction method for tripterygium glycosides
The invention discloses an extraction method for tripterygium glycosides. The method comprises the following steps of: (1) sample pretreatment: cleaning and smashing tripterygium wilfordii Hook. f. roots to obtain a powder sample; (2) crude product extraction: adding the powder sample into 50-60% ethyl alcohol at a solid-liquid ratio of 1:15-25, carrying out ultrasound extraction for 70-100 min under a condition of 200-300 W and 60-75 DEG C, and concentrating an extraction solution to 15-25% of the volume; and (3) crude product purification: adding a concentrate obtained in S (2) into chloroform at a solid-liquid ratio of 1:1-2, carrying out microwave extraction for 90-120 min under a condition of 280-380 W and 60-62 DEG C, and concentrating the extraction solution until specific gravity is 1.2-1.6. According to the method, the use amount of harmful organic solvents can be greatly lowered, an extraction rate of the tripterygium glycosides is obviously improved, the purity of effectiveingredients, including triptolide, triptonide, tripterine and wilforlide A, can be obviously improved, in addition, the extraction method is convenient in an extraction operation and can be easily controlled, and industrial production efficiency is improved.
Owner:HUNAN QIANJIN XIELI PHARMA CO LTD
Preparation of 2s-cardiospermin-5-benzoate and its application in the preparation of drugs for treating rheumatoid arthritis
ActiveCN106977560BHighlight substantive featuresOrganic active ingredientsSugar derivativesPharmaceutical SubstancesPharmacology
Owner:BINZHOU MEDICAL COLLEGE
Dry coating sustained-release tablet for treating arthritis and preparation process thereof
InactiveCN1969929AEasy to takeComplies with the law of toxic releaseOrganic active ingredientsAntipyreticSustained Release TabletControlled Release Tablet
The invention discloses a kind of dry dressed controlled release tablets for treating arthritis which comprises medicinal cores and dry coating sheet, wherein the dry coating sheet comprises slow release matrix material 110-150 parts, hole-making agent 100-120 parts, right amount of binding agent and magnesium stearate. The medicinal cores comprise Tripterygium wilfordii glycosides 20-30 parts, crumbling agent 10-20 parts, bonding agent 20-30 parts, thinning agent 30-40 parts, and right amount of magnesium stearate.
Owner:SOUTHERN MEDICAL UNIVERSITY
Application of tripterygium glycosides tablet in preparation of drugs for treating CAR-T induced cytokine release syndrome
ActiveCN111840355AReduce clinical treatment costsQuick containmentAntipyreticAnalgesicsT cellCytokine
The invention discloses an application of a tripterygium glycoside tablet in preparation of a medicine for treating CAR-T induced cytokine release syndrome. The research finds that the tripterygium glycosides tablet taking the tripterygium glycosides as the main component can be used for rapidly restraining IL-6, IL-8, IL-2, IL-10, IL-1 beta, IFN alpha, IFN gamma and the like, the tripterygium glycosides tablet is orally taken at a low dosage, so that activated mononuclear cells of peripheral blood can be selectively removed, the CRS generated after CAR-T treatment is rapidly restrained, in-vivo CAR-T cells are not affected, a rapid, effective, safe and convenient treatment method can be possibly provided for the CRS, meanwhile, a good cost-benefit ratio is achieved, and the clinical treatment cost of a patient can be greatly reduced. In addition, a positive treatment effect is expected to be achieved on the CRS of a critical patient infected by the novel coronavirus (COVID-19).
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Application of trillium tschonoskii total saponins in preparation of medicine for treating arthritis
InactiveCN113318176AReduce secretionInhibit progressAntipyreticAnalgesicsKnee JointInterstitial edema
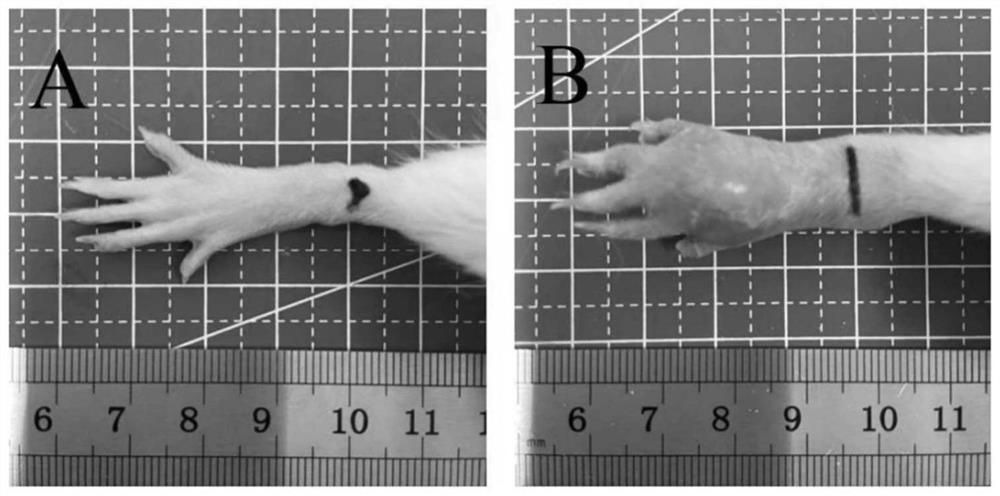
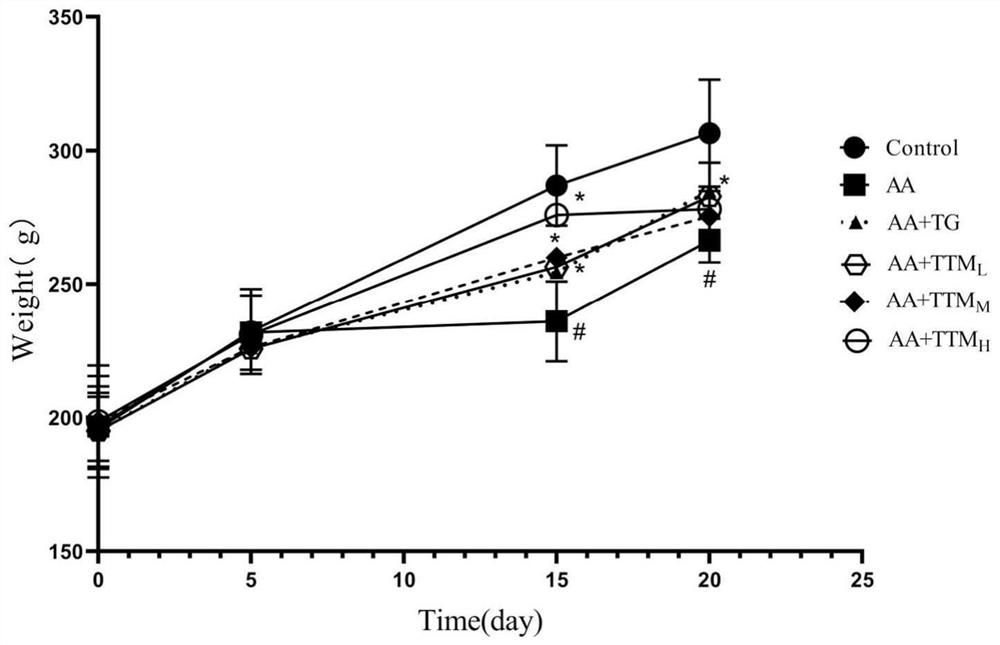
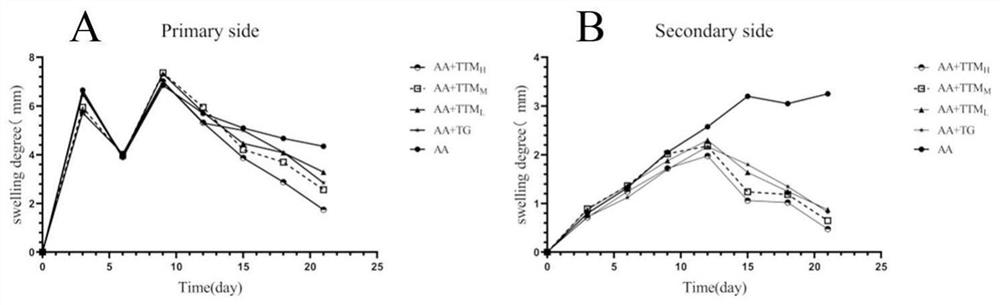
The invention provides an application of trillium tschonoskii total saponins in preparation of a medicine for treating rheumatoid arthritis. The effect of the trillium tschonoskii total saponins on adjuvant-induced arthritis model rat inflammation is researched from an NOD-like receptor protein 3 / cysteine aspartic protease 1 pathway. The SD rats are randomly divided into a blank group, a model group, a tripterygium glycosides group, a trillium tschonoskii total saponin low-dose group, a trillium tschonoskii total saponin medium-dose group and a trillium tschonoskii total saponin high-dose group, and each group comprises six rats. The result shows that compared with the Control group, the foot swelling degree, the arthritis score, the serum TNF-alpha, IL-1beta and IL-6 levels of the AA group, the NLRP3 protein expression level in synovial tissues and the NLRP3, ASC and Caspase-1 protein levels in ankle joints are all remarkably improved; knee joint synovial cell proliferation, interstitial edema and inflammatory cell infiltration of the AA group rats; and after treatment with TG and TTM with different doses, the indexes are all improved to different degrees.
Owner:CHINA THREE GORGES UNIV
Medicinal uses of capers or caper extracts
ActiveCN109303790BImprove protectionSignificant ethyl acetate extract effectOrganic active ingredientsAntimycoticsLeprosyRenal glomerulus
The present invention relates to the medical use of Capers or Capers extracts, specifically provides Capers or Capers extracts in the preparation of medicines for reducing tripterygium wilfordii, tripterygium glycosides or triptolide hepatotoxicity or The application in health products also provides a composition and its preparation for preventing and treating rheumatoid arthritis, glomerulonephritis, nephrotic syndrome, lupus erythematosus, dry eye syndrome, Behcet's disease, eczema, psoriasis , leprosy, scabies, ringworm, Hashimoto's thyroid or tumor medicine or health care products, the composition comprises component i) and component ii), and the component i) is selected from: Capers or the extract of capers; the component ii) is selected from: tripterygium glycosides or triptolide.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Preparation of 2s-cardiospermin-5-cis-p-coumarate and its application in the preparation of rheumatoid arthritis drugs
ActiveCN106974922BHighlight substantive featuresOrganic active ingredientsSugar derivativesPharmaceutical SubstancesPharmacology
The invention relates to a chemical structure and preparing method of the compound 2S-cardiospermin-5-cis-p-coumarate and application of the compound to preparation of drugs for treating rheumatoid arthritis. 2S-cardiospermin-5-cis-p-coumarate is a novel compound prepared from the traditional Chinese medicine Sorbaria sorbifolia (L.) A. Brown. through purification by the applicant of the invention. The compound can remarkably suppress the level of PEG2 and NO in serum of adjuvant arthritis rats. The activity of the compound is close to that of tripterygium glycosides of the same dose, and the compound can be used for preparing drugs for treating rheumatoid arthritis. The chemical structure, preparing method and anti-RA activity of the compound are disclosed for the first time, thus having prominent and substantive features.
Owner:BINZHOU MEDICAL COLLEGE
A kind of method for reducing organic solvent residue in tripterygium glycosides
The invention relates to a method for reducing residual organic solvents in tripterygium glycosides. In the production process of tripterygium glycosides, organic solvents (mainly ethanol and chloroform) must be used for extraction, extraction, chromatography, etc. Currently, there is a problem of unstable residual indicators of organic solvents in tripterygium glycosides. The present invention dissolves the semi-finished tripterygium glycosides with absolute ethanol, and fully stirs to make the dissolution uniform; concentrates the obtained tripterygium glycosides ethanol liquid, recovers the ethanol, and decompresses and drains to obtain a powdery dry product; then uses warm water to precipitate Combined with the low-temperature vacuum drying method, the tripterygium glycosides finished product is obtained. The invention can effectively reduce the residual organic solvent in the tripterygium glycosides, and has stable quality.
Owner:浙江得恩德制药股份有限公司
Combined application of phellinus igniarius and tripterygium glycosides in delaying focal segmental glomerulosclerosis process
ActiveCN111514179APlay a role in synergizing and reducing toxicityEasily damagedMetabolism disorderFungi medical ingredientsCreatine kinaseRenal glomerulus
The invention discloses an application of phellinus igniarius and tripterygium glycosides in delaying a focal segmental glomerulosclerosis process. The combined phellinus igniarius and tripterygium glycosides can significantly reduce urine protein, reduce serum creatinine and urea nitrogen of rats, reduce serum triglyceride, cholesterol and low-density lipoprotein, and reduce the amount of creatine kinase, creatine kinase isozyme and glutamic-pyruvic transaminase. The phellinus igniarius water decoction is combined with the tripterygium glycosides for use, so that the toxicity of the tripterygium glycosides can be reduced and the heart injury can be improved on the basis of ensuring the original effects of the tripterygium glycosides on relieving the renal function injury of FSGS rats, reducing blood fat and the like, and the application prospect is wide.
Owner:HANGHZOU HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Preparation of isocardiospermin-5-p-hydroxybenzoate and its application in the preparation of drugs for treating rheumatoid arthritis
ActiveCN106974924BHighlight substantive featuresOrganic active ingredientsSugar derivativesPharmaceutical SubstancesHydroxybenzoate
The invention relates to a chemical structure and preparing method of the compound isocardiospermin-5-p-hydroxybenzoate and application of the compound to preparation of drugs for treating rheumatoid arthritis. Isocardiospermin-5-p-hydroxybenzoate is a novel compound prepared from the traditional Chinese medicine Sorbaria sorbifolia (L.) A. Brown. through purification by the applicant of the invention. The compound can remarkably suppress the level of PEG2 and NO in serum of adjuvant arthritis rats. The activity of the compound is close to that of tripterygium glycosides of the same dose, and the compound can be used for preparing drugs for treating rheumatoid arthritis. The chemical structure, preparing method and anti-RA activity of the compound are disclosed for the first time, thus having prominent and substantive features.
Owner:BINZHOU MEDICAL COLLEGE
Application of tripterygium glycoside tablet in preparation of medicine for relieving and/or treating hemophagocytic syndrome related symptoms
ActiveCN111956680ACurb the StormReduce clinical treatment costsPill deliveryBlood disorderSide effectCytokine
The invention discloses a novel medical application of a tripterygium glycoside tablet, and particularly discloses an application of the tripterygium glycoside tablet in preparation of a medicine forrelieving and / or treating hemophagocytic syndrome related symptoms. Researches prove that the tripterygium glycoside tablet can significantly relieve the hemophagocytic syndrome related symptoms of patients, namely, can remarkably relieve repeated high fever, obviously relieve abdominal distension, relieve splenomegaly, reduce ascites and rapidly reduce the content of soluble CD25 in blood, and also has significant curative effects on recovery of hematopoietic functions and coagulation functions. The orally taken tripterygium glycoside tablet can rapidly inhibit cytokine storm and rapidly relieve various hemophagocytic syndrome related symptoms, has small toxic and side effects, can provide conditions for subsequent treatment including chemotherapy and hematopoietic stem cell transplantation, has a good cost-benefit ratio, and can greatly reduce the clinical treatment cost of the patients.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Application of total flavonoids of clover in preparation of medicine for treating rheumatoid arthritis
ActiveCN106913610BEffective in treating rheumatoid arthritisEasily damagedAntipyreticAnalgesicsRheumatismArthritis
The invention relates to the technical field of medicines for conditioning rheumatoid arthritis, and discloses an application of total flavonoids of radix tetrastigme in rheumatoid arthritis treating medicines. The medicines are capable of conditioning the rheumatoid arthritis. A preparation process of the total flavonoids of radix tetrastigme comprises the following steps: prpearing 10-30kg of coarse powder of tubers of radix tetrastigme; conducting reflux extraction for 3 times with the addition of ethanol which is 65-75% in volume percentage; combining extracting solutions, decompressing the combined extracting solutions and recovering ethanol; keeping an obtained solution for a whole night and filtering the solution; extracting an obtained filtrate for three times by virtue of petroleum ether and conducting degreasing; processing the greased filtrate by virtue of a polyamide column; eluting the column sequentially by virtue of water and ethanol which is 90-95% in volume percentage; and decompressing an eluent of the ethanol which is 90-95% in volume percentage and recovering ethanol until a dried material is obtained, namely the total flavonoids of radix tetrastigme. At present, tripterygium glycosides, as a traditional Chinese medicine, can take a relatively good effect on treating the rheumatoid arthritis, however the tripterygium glycosides can cause various toxic and side effects, such as relatively serious injury to female ovaries and male sperms, and subsequently, clinical application is limited. According to the invention, the total flavonoids of radix tetrastigme, on treating the rheumatoid arthritis, is good in effect and free from toxic and side effects.
Owner:曹岗 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com