Application of tripterygium glycosides tablet in preparation of drugs for treating CAR-T induced cytokine release syndrome
A technology of cytokines and polyglycosides, applied in the field of medicine, can solve problems such as unclear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
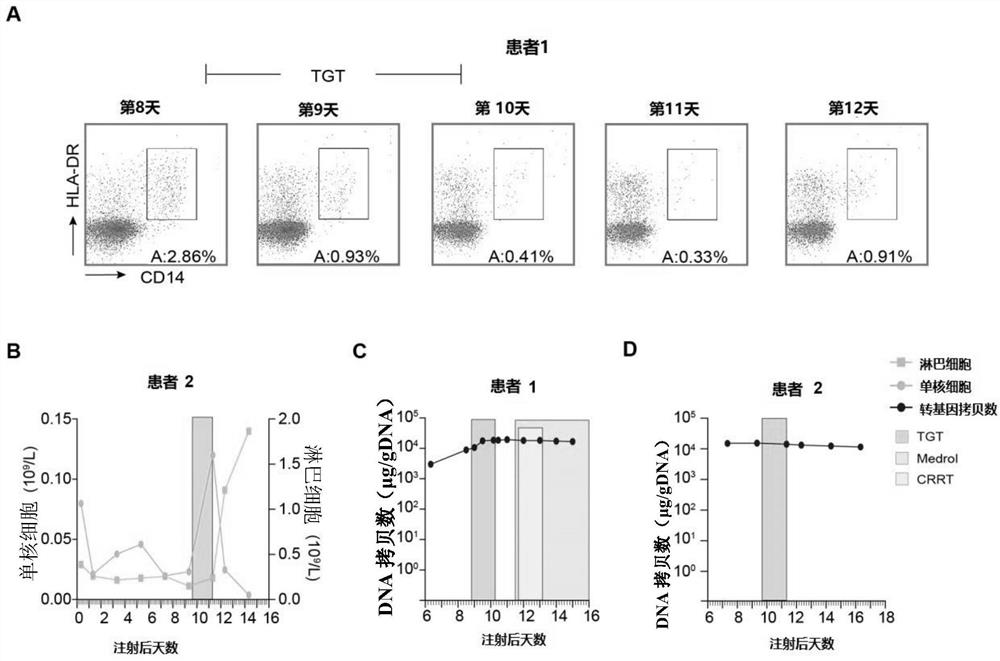
[0022] Example 1 Tripterygium glycosides tablets selectively cleared peripheral blood mononuclear cells of patients in vivo without affecting CAR-T cells.
[0023] 1) Two patients diagnosed with CRS after CAR-T cell infusion were given oral low-dose tripterygium glycosides tablets, three times a day, 10 mg each time (total daily dose 0.5 mg / kg), within 36 hours After taking 5 tripterygium glycosides tablets (50 mg), the drug was stopped.
[0024] 2) Take anticoagulant peripheral blood at different time points before and after taking the medicine for routine testing. In addition, take 200 μl of anticoagulant peripheral blood, add 5ml flow cytometry tubes, add fluorescently labeled antibodies HLA-DR-PE, CD14-FITC, and incubate on ice for 30 minutes , after lysing red blood cells, flow cytometry detection.
[0025] 3) qPCR determination of CAR-T cell copy number: EDTA anticoagulated whole blood samples were collected at different time points before and after taking the drug, and...
Embodiment 2
[0031] Example 2 Tripterygium glycosides quickly suppressed CRS.
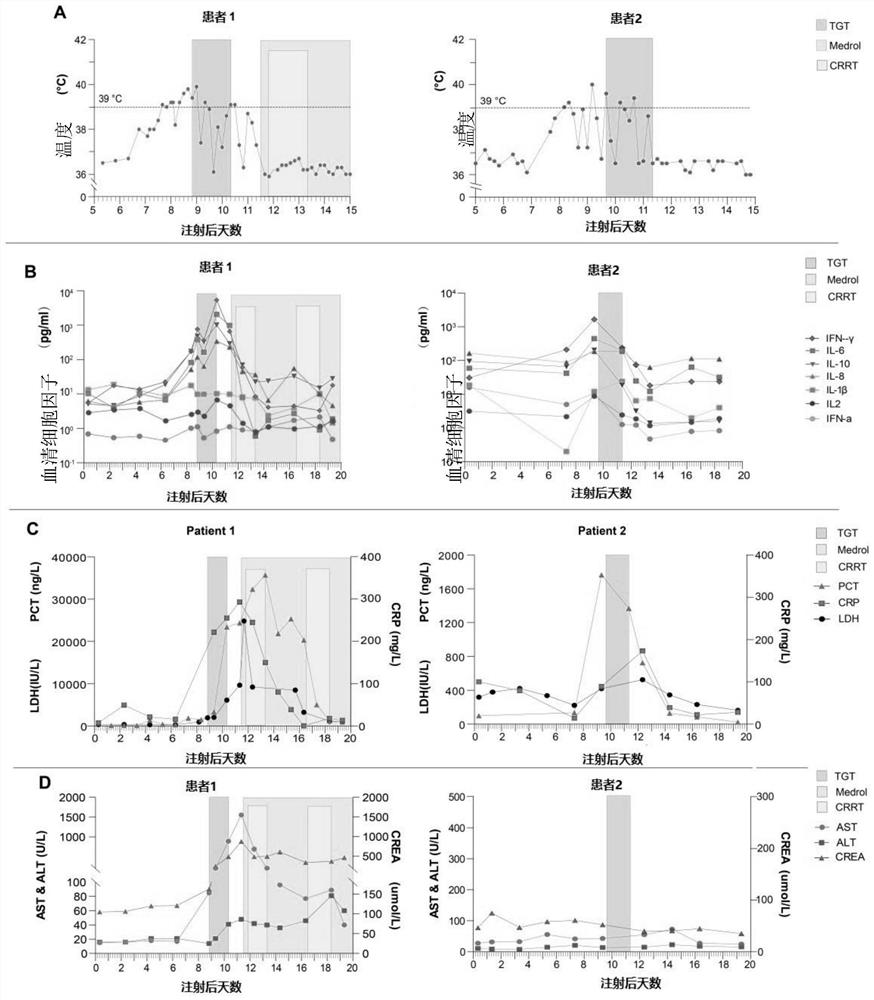
[0032]Patient 1 was diagnosed with multiple myeloma (IgD, lambda type) in 2017, aged 53 years. His baseline karyotype is 46, XY, +add(1)(p11), -3, del(3)(q13), add(9)(q34), del(11)(p12), add(14) (q32), -17, 18, add(20)(q11), add(22)(q11), +2mar, inc[20]. The patient had an initial partial response to treatment with lenalidomide, bortezomib, and dexamethasone, but his disease worsened after 6 months. Then received 3 cycles of VCLD chemotherapy (bortezomib, cyclophosphamide, lenalidomide and dexamethasone). Subsequent regimens included lenalidomide, bomalidomide, melphalan, vinblastine, epirubicin, and arsenous acid. The patient's bone destruction and anemia worsened, and a bone marrow biopsy showed 52% multiple myeloma cells. In November 2019, the patient participated in a clinical trial of anti-CD19 / BCMA-CAR-T cell therapy. The patient's autologous T cells were transfected with lentivirus to express CD19-s...
Embodiment 3
[0036] Example 3 The treatment of Tripterygium wilfordii Polyglycoside Tablets rapidly suppresses CRS.
[0037] Patient 2 in this example is a 55-year-old female who was diagnosed with B-ALL in 2017. The patient was in complete remission after starting treatment but relapsed 15 months after the initial diagnosis of B-ALL. She did not respond to re-chemotherapy and further intensive chemotherapy, including vindesine, epirubicin, and cyclophosphamide. The patient participated in a clinical trial of anti-CD19-CAR-T cell therapy. The patient's autologous T cells were transfected with lentivirus to express CD19-specific chimeric antigen receptors. Two days prior to cell infusion, the patient was pretreated with chemotherapy (fludarabine at a dose of 30 mg / m 2 Body surface area, cyclophosphamide dose is 600mg / m 2 ), and then the patient was infused with 1.31×10 6 CD3+ cells (0.52×10 per kg 6 CD19-CART cells).
[0038] On the 7th day after the reinfusion of CAR-T cells, the pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com