Nintedanib supramolecular co-loaded clathrate compound
A technology of nintedanib and nintedanib ethanesulfonate, which is applied in the directions of drug combinations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 nintedanib supramolecular clathrate
[0035]
[0036] Weigh HP-β-CD, add appropriate amount of water, stir to dissolve; add nintedanib ethanesulfonate, continue stirring to dissolve nintedanib ethanesulfonate; add NaOH solution to adjust pH 5.0-7.5, add water to Sufficient amount is stirred evenly to prepare nintedanib supramolecular clathrate. Among them, nintedanib ethanesulfonate / hydroxypropyl-β-cyclodextrin (10:200) or more, the inclusion rate is greater than 90%.
Embodiment 2
[0037] Example 2 Preparation of nintedanib / itraconazole co-loaded inclusion compound
[0038]
[0039] Weigh itraconazole, add cyclodextrin derivatives and appropriate amount of water, stir to completely dissolve itraconazole; then add nintedanib ethanesulfonate, continue to stir to completely dissolve; adjust pH with sodium hydroxide solution value to 5.0-7.5, and add water to a sufficient amount to obtain nintedanib / itraconazole co-loaded clathrate solution.
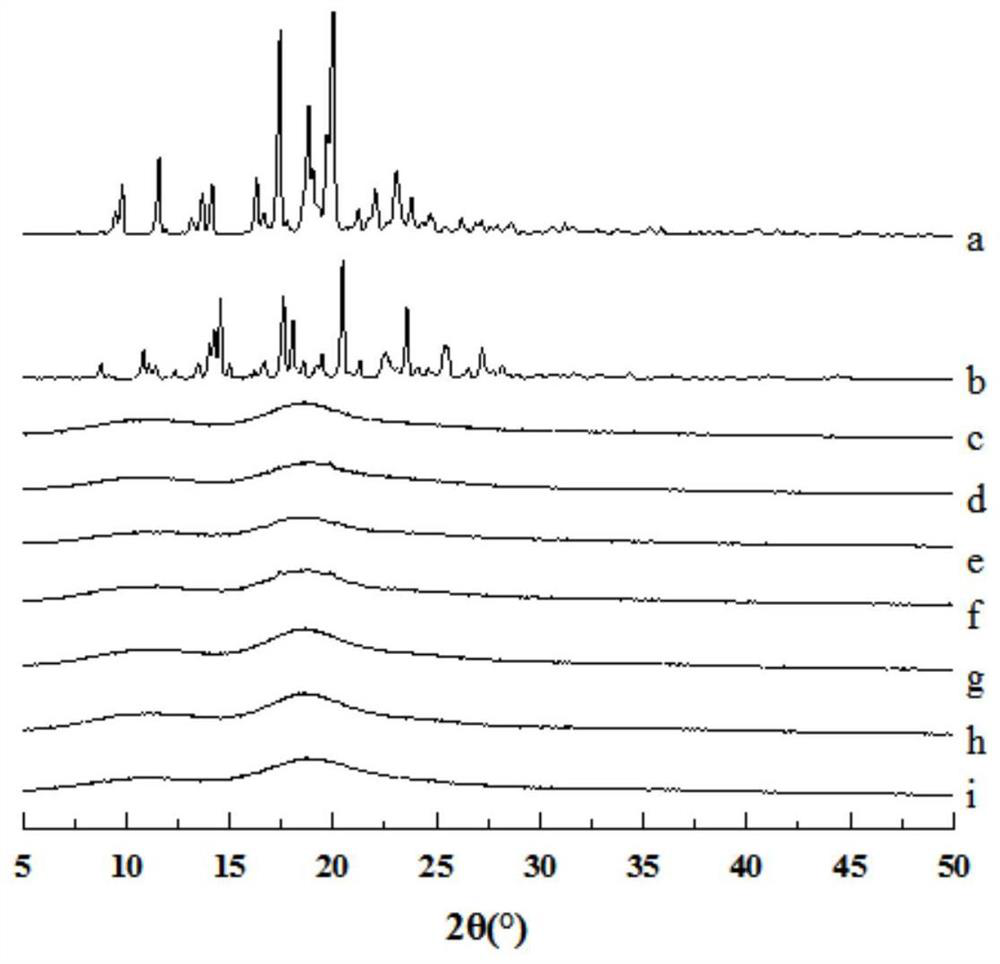
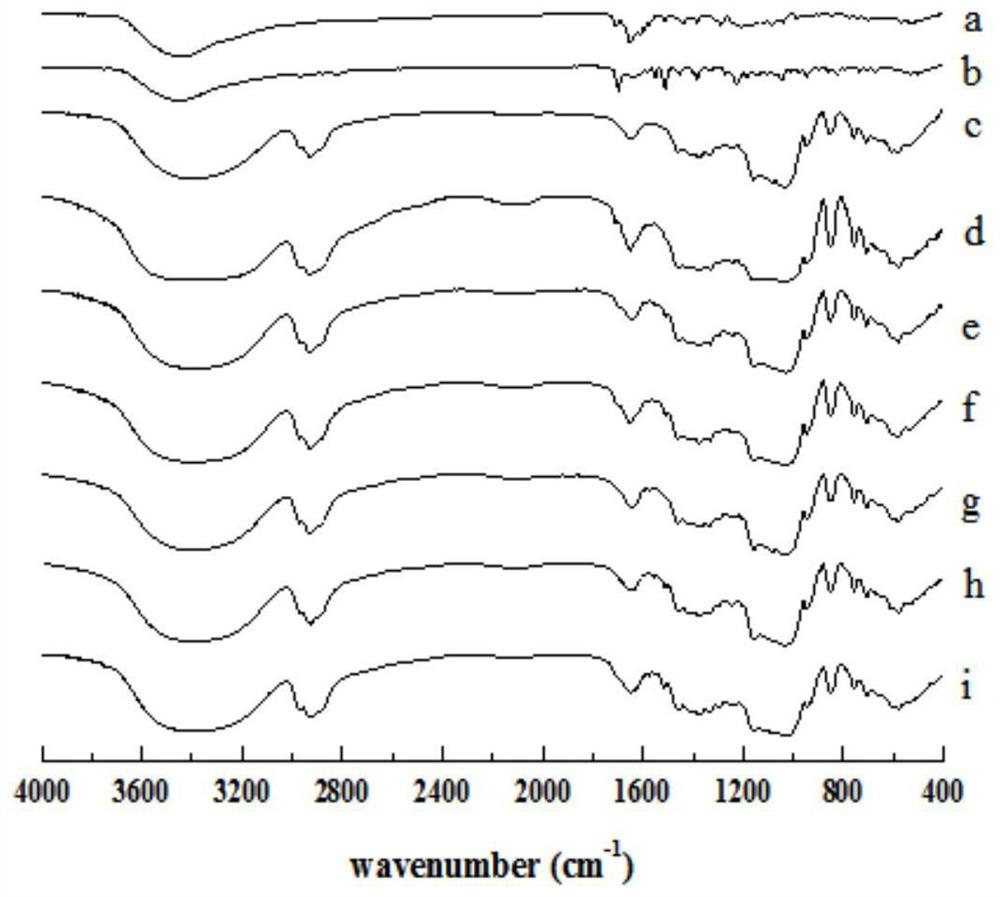
[0040] XRD and infrared images showed that both nintedanib ethanesulfonate and itraconazole existed in the co-carrier molecular clathrate in molecular form.
Embodiment 3
[0041] Example 3 Oral pharmacokinetics of nintedanib inclusion compound combined with itraconazole
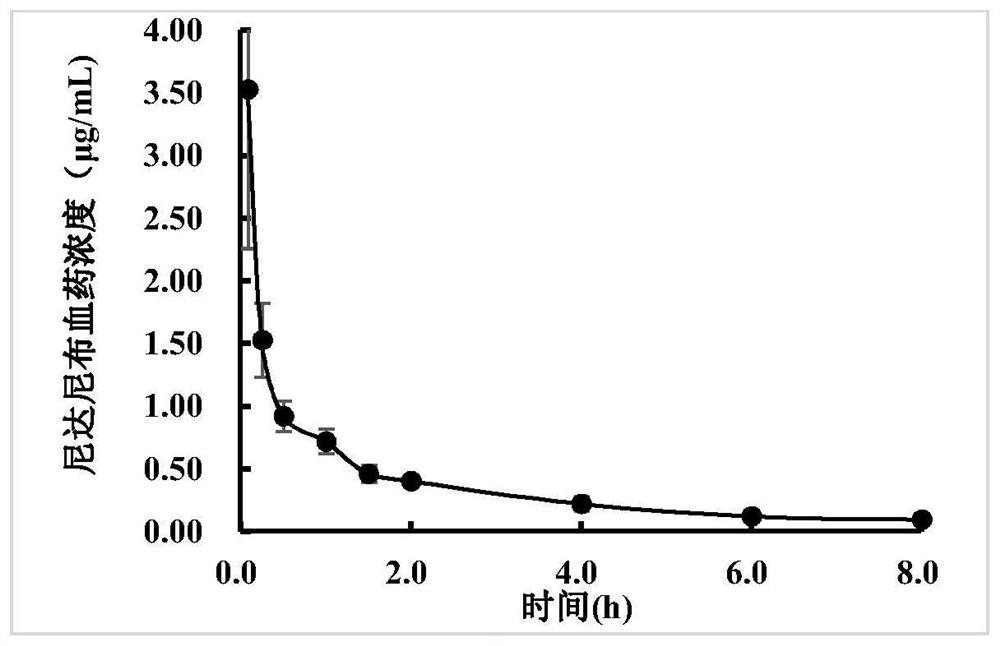
[0042] Commercially available itraconazole capsule contents (dose 50mg / kg) rat gavage, give prescription 4 and prescription 6 nintedanib inclusion compound (dose 50mg / kg) preparation in embodiment 1 again by gavage after 30min, Determination of blood concentration. attached image 3 It is the drug-time curve of nintedanib injection after tail vein injection in rats. attached Figure 4The drug-time curves of prescription 4 and prescription 6 nintedanib inclusion compound and commercially available itraconazole capsule content or itraconazole inclusion compound intragastric administration to rats, compared with prescription 6 alone, and nintedanib Cloth solution was compared with the contents of commercially available itraconazole capsules.
[0043] The results show that with Figure 4 In schemes 1#, 2#, 3#, 4# and 5#, the absolute bioavailability of nintedanib was 12.2%, 23...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com