Dry coating sustained-release tablet for treating arthritis and preparation process thereof
A controlled-release tablet and arthritis technology, which is applied in the field of traditional Chinese medicine preparations, can solve the problems of drug bioavailability reduction, enhancement of drug side effects, and strengthening, so as to reduce the number of administrations and doses, reduce the amount of tripterygium glycosides, reduce The effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
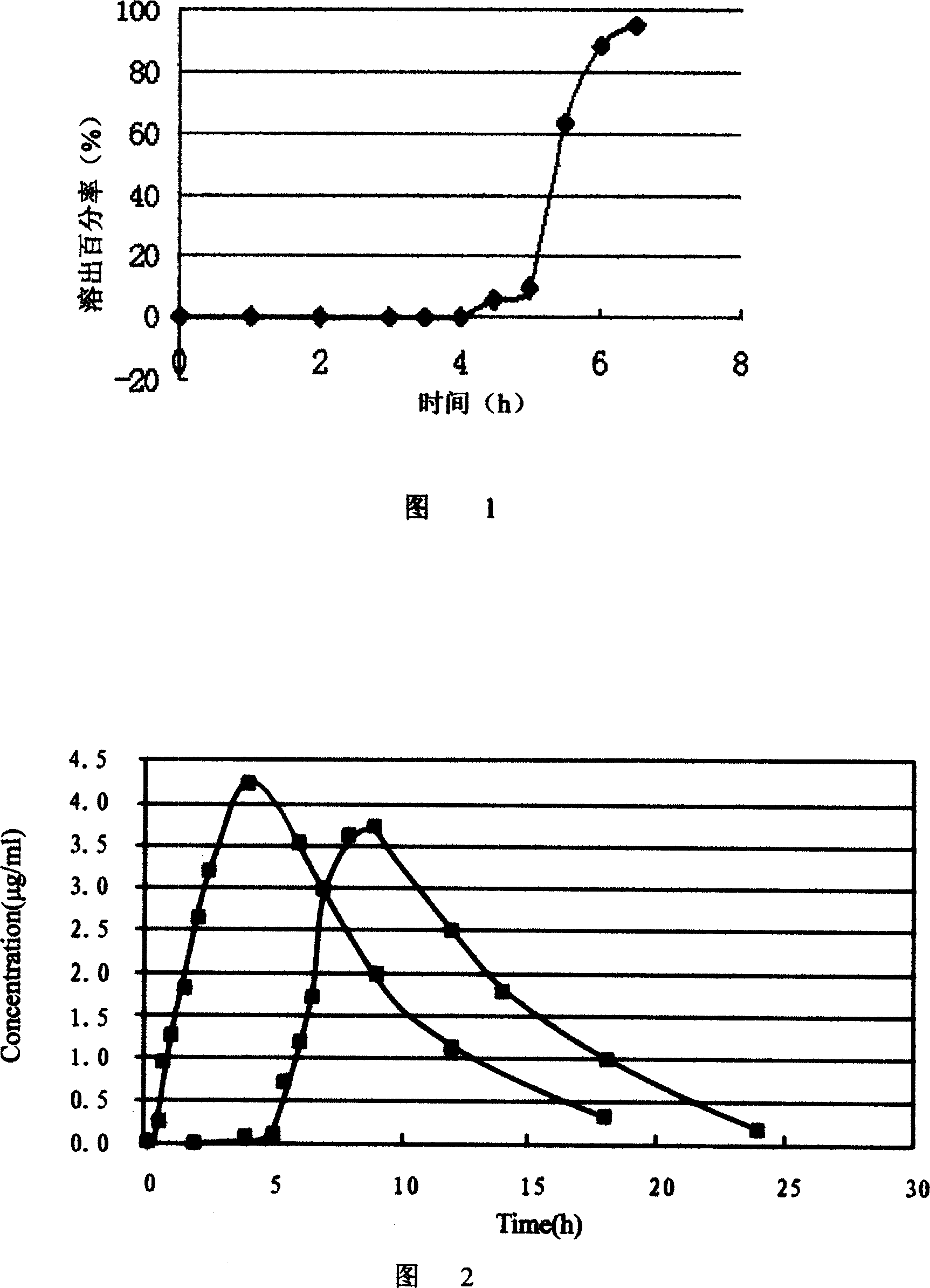
Examples
Embodiment 1
[0034] 1. Preparation prescription:
[0035] Dry coating: 110 parts of PEG6000, 110 parts of hydrogenated castor oil (HCO), 15 parts of ethyl cellulose (EC).
[0036] Drug tablet core: 25 parts of tripterygium glycosides, 15 parts of microcrystalline cellulose, 35 parts of pregelatinized starch, 25 parts of methyl cellulose, appropriate amount of magnesium stearate, and appropriate amount of lactose.
[0037] 2. Preparation method:
[0038] 1), weigh tripterygium glycosides, microcrystalline cellulose, pregelatinized starch, methyl cellulose and lactose according to the prescription amount (the loss should be increased according to the size of the preparation amount), pass through a 60 mesh sieve and mix three times, add pregelatinized starch A mixed binder of gelatinized starch and methyl cellulose is used to make soft materials. Pass through a 20-mesh sieve to granulate, and dry at 55°C for 60 minutes. Pass through a 20-mesh sieve for granulation. Add an appropriate amou...
Embodiment 2
[0042] 1. Preparation prescription:
[0043] Dry coating: 120 parts of PEG6000, 110 parts of hydrogenated castor oil (HCO), 15 parts of ethyl cellulose (EC).
[0044] Drug tablet core: 25 parts of tripterygium glycosides, 15 parts of microcrystalline cellulose, 35 parts of pregelatinized starch, 25 parts of methyl cellulose, appropriate amount of magnesium stearate, and appropriate amount of lactose.
[0045] 2. Preparation method:
[0046] 1), weigh tripterygium glycosides, microcrystalline cellulose, pregelatinized starch, methyl cellulose and lactose according to the prescription amount (the loss should be increased according to the size of the preparation amount), pass through a 60 mesh sieve and mix three times, add pregelatinized starch A mixed binder of gelatinized starch and methyl cellulose is used to make soft materials. Pass through a 20-mesh sieve to granulate, and dry at 55°C for 60 minutes. Pass through a 20-mesh sieve for granulation. Add an appropriate amou...
Embodiment 3
[0050] 1. Preparation prescription:
[0051] Dry coating: 110 parts of PEG6000, 100 parts of hydrogenated castor oil (HCO), 10 parts of ethyl cellulose (EC).
[0052] Drug tablet core: 30 parts of tripterygium glycosides, 10 parts of microcrystalline cellulose, 30 parts of pregelatinized starch, 30 parts of methyl cellulose, appropriate amount of magnesium stearate, and appropriate amount of lactose.
[0053] 2. Preparation method:
[0054] 1), weigh tripterygium glycosides, microcrystalline cellulose, pregelatinized starch, methyl cellulose and lactose according to the prescription amount (the loss should be increased according to the size of the preparation amount), pass through a 60 mesh sieve and mix three times, add pregelatinized starch A mixed binder of gelatinized starch and methyl cellulose is used to make soft materials. Pass through a 20-mesh sieve to granulate, and dry at 55°C for 60 minutes. Pass through a 20-mesh sieve for granulation. Add an appropriate amou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com