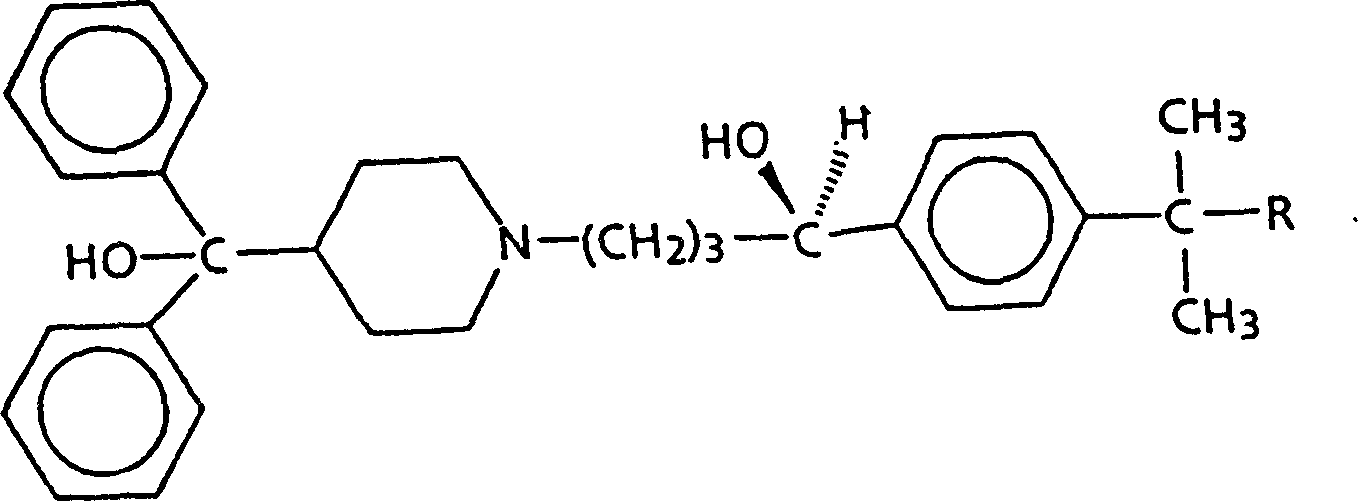
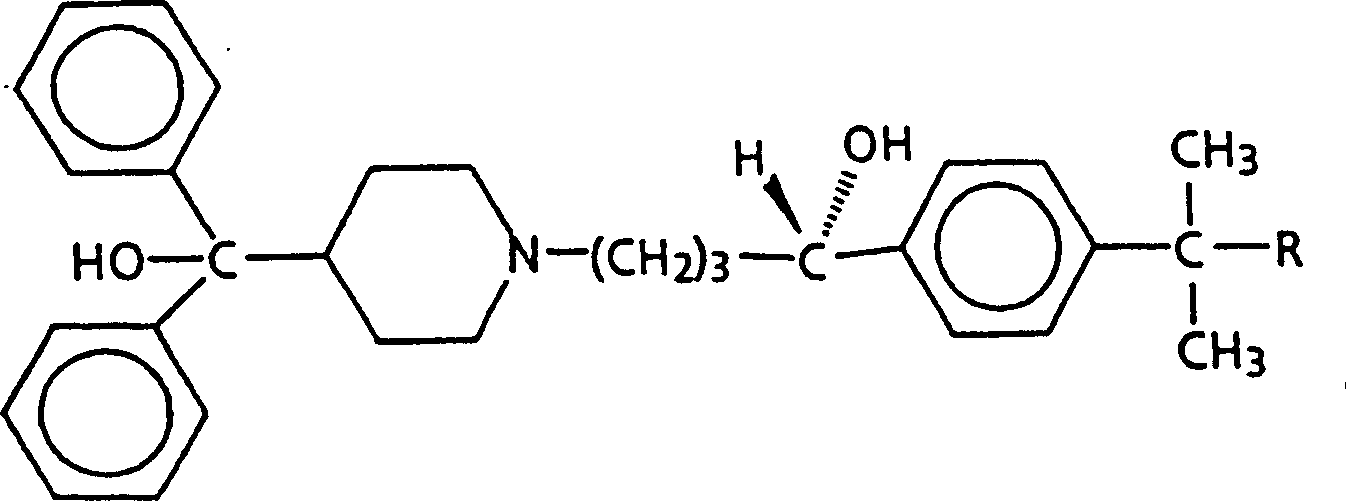
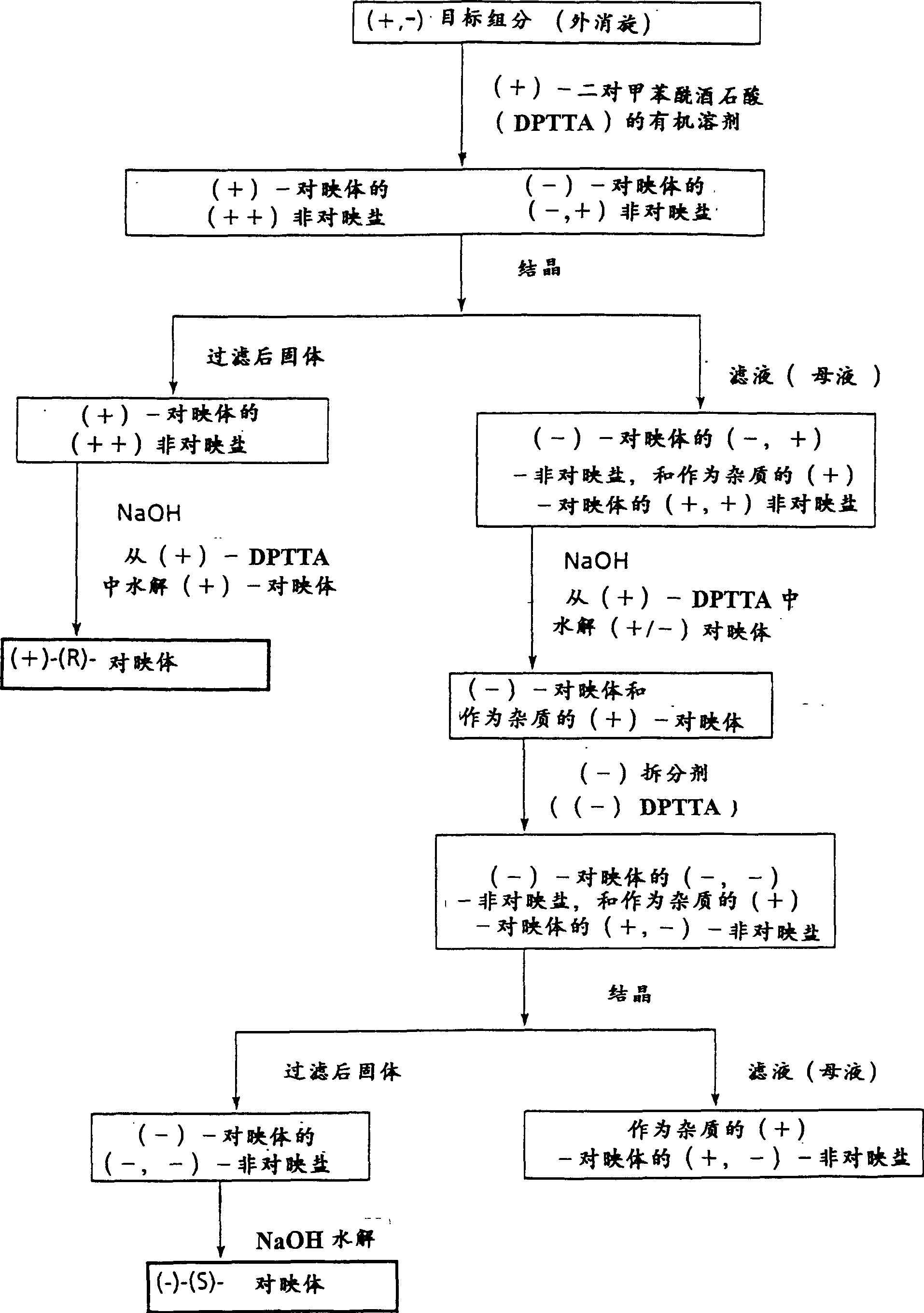
Diastereomer salts of terfenadine
A technology of diastereomer and piperidinol is applied in the field of separation of racemic components, and can solve the problems of infeasible application of chiral separation reagents on an industrial scale.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0101] Embodiment 1A (R)-(+)-terfenadine
[0102] Racemic α-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1-piperidinebutanol (terfenadine) ( 10.0 g, 21.2 mmol) and (2S, 3S)-(+)-di-p-toluoyl tartaric acid monohydrate ((+)-DPTTA) (8.60 g, 21.3 mmol) were dissolved in 90 ml of acetone. The resulting solution was cooled to room temperature (15°C to 30°C) for one day and then placed in the refrigerator for another day. The resulting crystals were collected by filtration, and the precipitated diastereomeric salts contained (+)-terfenadine and (+)-DPTTA (chemical yield 98%, diastereomeric salt excess 90% (%de)).
[0103] The salt was recrystallized twice from about 8 mL of acetone (per gram of salt) and dried in vacuo at 80°C for one day to give the purified diastereomeric salt (7.54 g, 83% chemical yield, about 100%de) . The melting point is about 125-134°C (hot stage).
[0104] IR(KBr): 2800-2200, 1720, 1610, 1265, 1105cm -1 .
[0105] [α] D 24 +20° (c=1.0, CHCl...
Embodiment 1B
[0117] Embodiment 1B (S)-(-)-terfenadine
[0118] To the crystallization mother liquor of the diastereomeric salts (R)-(+)-terfenadine and (2S,3S)-(+)-di-p-toluoyltartaric acid was added 22 ml of 1N sodium hydroxide and 80 ml of water . The resulting crystals were collected and recrystallized once from ethanol / water to give partially resolved (S)-(-)-terfenadine in 96% chemical yield.
[0119] The crystals were combined with (2R, 3R)-(-)-di-p-toluoyl tartaric acid (3.94 g, 10.2 mmol) in 75 ml of acetone in equimolar proportions, left at room temperature (15°C to 30°C) for one day, and then Put it in the refrigerator for another day. The resulting crystals were collected by filtration to give diastereomeric salts of (S)-(-)-terfenadine and (-)-di-p-toluoyltartaric acid. This salt was recrystallized once from about 8 mL of acetone (per gram of salt) and dried in vacuo at 80 °C for one day to give the purified diastereomeric salt (7.03 g, 77% chemical yield) with about 100% op...
Embodiment 2A
[0130] Embodiment 2A (R)-(+)-terfenadine
[0131] Racemic terfenadine (20 g, 42.4 mmol) and (R)-(-)-mandelic acid (6.45 g, 42.4 mmol) were dissolved in 180 mL of methanol by heating to about 60 °C . The resulting solution was cooled to room temperature (15°C to 30°C) for one day, and then placed in a 4°C refrigerator for another day. The resulting crystals were collected by vacuum filtration, and the resulting crystalline diastereomeric salts included the resolving agent and the (+)-enantiomer (chemical yield 101%, 78%de). The crystals were recrystallized twice from about 9 mL of methanol (per gram of salt) and dried in vacuo at 80°C for one day to give purified diastereomeric crystals (9.70 g, 73% chemical yield, 99%de). The melting point is about 112-118°C (hot stage).
[0132] IR(KBr): 2800-2100, 1610, 1360cm -1 .
[0133] [α] D 23 -5.9° (c=2.0, CHCl 3 )
[0134] Analysis: C 40 h 49 NO 5 :
[0135] Calculated value C: 77.01; H: 7.92; N: 2.25;
[0136] Measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com