Verification and separation method for terfenadine related substance
A technology of terfenadine and related substances, which is applied in the field of drug impurity testing, can solve problems such as not being able to fully and effectively achieve separation, not being able to fully meet the needs of use, and achieve good practical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for testing and separating related substances of terfenadine, the process is as follows:
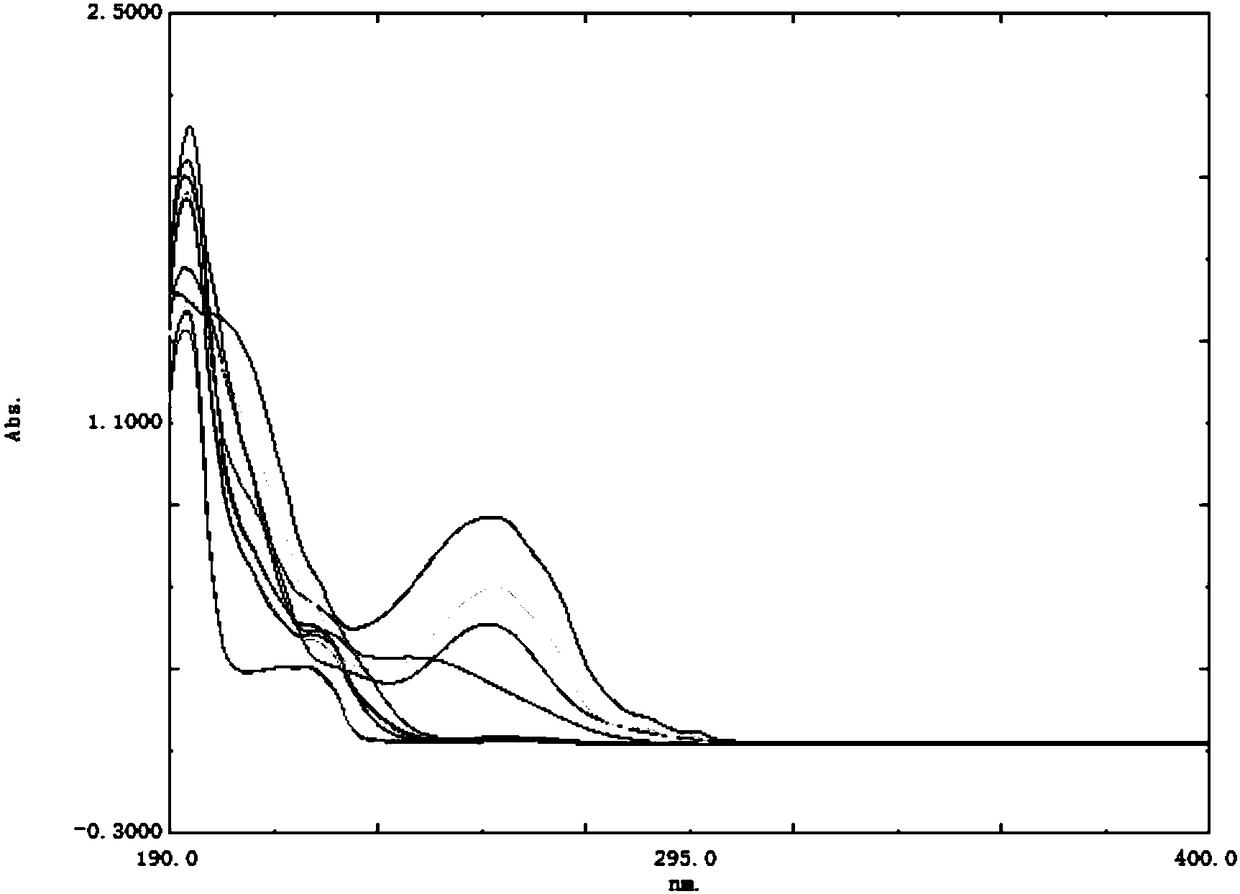
[0028] 1) Take appropriate amounts of impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, and TFND, and add 70% acetonitrile to make a single solution of 10 μg / mL, Carry out full-wavelength scanning at a wavelength of 190nm to 400nm to obtain the scanning wavelength of each substance, as shown in Table 1 and figure 1 shown.
[0029] Table 1 Wavelength selection result table
[0030]
[0031]
[0032]
[0033] Depend on figure 1 It can be seen that the UV absorption of impurities A, B, C, D, E, F, G, H, I, J, and terfenadine are quite different, but in the detection method specified in the EP standard There is a large absorption at the wavelength (217nm), and the detection wavelength is set at 217nm in combination with the quality standards of the European Pharmacopoeia and the results of ultra...
Embodiment 2
[0039] The inspection separation method of terfenadine related substance, with embodiment 1, wherein, the chromatographic condition in step 2) is as follows:
[0040] Chromatographic column: MZ-ANALYTICAL Column 250*4.6mm 100ODS-3 5μm. Mobile phase A: acetonitrile-phosphate (dissolve 3.58g disodium hydrogen phosphate dodecahydrate in 1000mL water, adjust the pH to 6.0 with phosphoric acid, then add 1.92g SDS to dissolve and filter) = 20︰80; mobile phase B: acetonitrile. Gradient elution: 0min, 60%A, 40%B; 3min, 55%A, 45%B; 20min, 20%A, 80%B. Solvent: acetonitrile-phosphate=45:55. Wavelength: 217nm. Flow rate: 1.0 mL / min. Column temperature: 35°C. Injection volume: 20 μL.
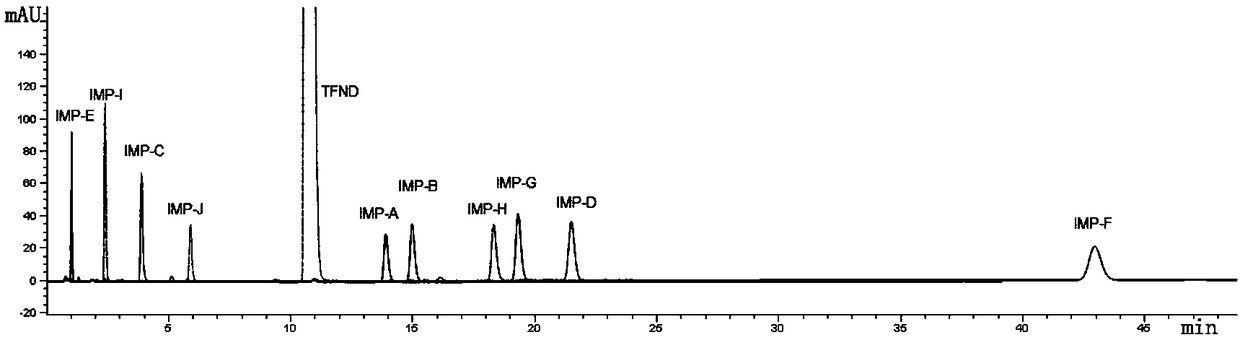
[0041] Inject the mixed solution and record the chromatogram, such as image 3 As shown, the results show that the peak eluting time of each impurity under this condition is reasonable, but the unknown impurity and impurity H cannot be separated well.
Embodiment 3
[0043] The inspection separation method of terfenadine related substance, with embodiment 2, wherein, the chromatographic condition in step 2) is as follows:
[0044] Chromatographic column: MZ-ANALYTICAL Column 250*4.6mm 100ODS-3 5μm. Mobile phase A: acetonitrile-phosphate (dissolve 3.58g disodium hydrogen phosphate dodecahydrate in 1000mL water, adjust the pH to 6.0 with phosphoric acid, then add 1.92g SDS to dissolve and filter) = 20︰80; mobile phase B: acetonitrile-methanol = 95:5. Gradient elution: 0min, 40%A, 60%B; 10min, 40%A, 60%B; 20min, 20%A, 80%B. Solvent: buffer salt (pH6.0)-acetonitrile=40:60. Wavelength: 217nm. Flow rate: 1.0 mL / min. Column temperature: 30°C. Injection volume: 20 μL.
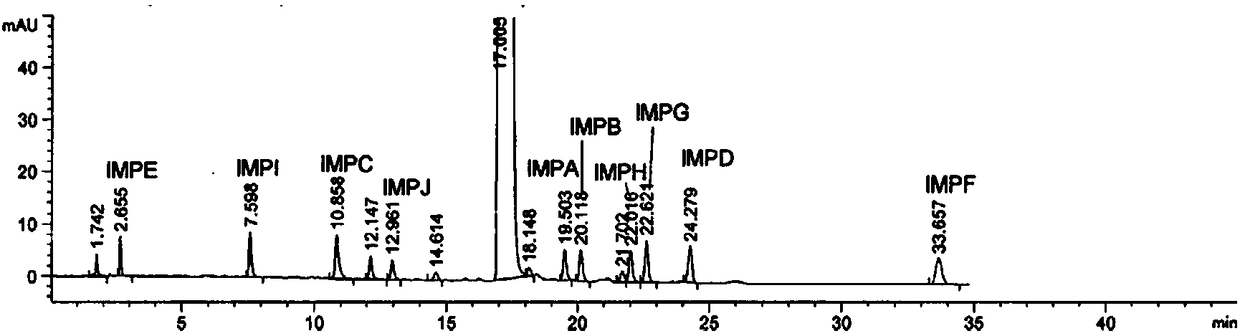
[0045]Take the mixed solution 2 (prepared with the mixed solution 1 of Example 1, add solvent (buffer salt-acetonitrile=40: 60) to prepare about impurity A, impurity B, impurity C, impurity D, impurity E, impurity F per 1mL , impurity G, impurity H, impurity I, impurity J ea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com