Bilastine-containing pharmaceutical composition and preparation method thereof
A bilastine and composition technology, applied in the field of pharmaceutical preparations, can solve the problems of sensitization, toxicity and the like, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
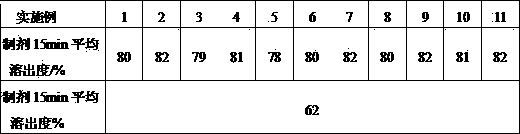
[0021] Bilastine 5% microcrystalline cellulose 64% Sodium carboxymethyl starch 26.7% Colloidal silica 3% Magnesium stearate 1% total 100%
[0022] Preparation Process:
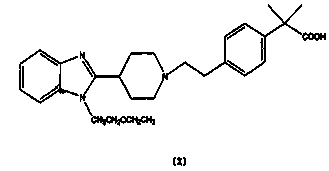
[0023] The active ingredient bilastine in the prescription is micronized, and the particle size is controlled at 5-50 μm. Mix the prescribed amount of bilastine with microcrystalline cellulose, sodium carboxymethyl starch, colloidal silicon dioxide, and magnesium stearate in a mixer. On a tablet press, compress the tablets.
Embodiment 2
[0025] Bilastine 13.3% lactose 57.2% microcrystalline cellulose 20% Crospovidone 8% Povidone K30 0.5% Magnesium stearate 1% total 100%
[0026] Co-micronize the active ingredient Bilastine and part of lactose in the prescription, and control the particle size of 90% of the volume or more particles to be in the range of 5-50 μm, so the excipients are passed through a 100-mesh sieve, and the micronized product and The remaining lactose, microcrystalline cellulose, and cross-linked povidone are mixed evenly by the method of equal addition, and an appropriate amount of PVP K30 is added to make a soft material, which is extruded through a 18-mesh sieve to granulate, and the wet granules are dried at a constant temperature at 50°C After 2 hours, the dry granules were sieved with a 24-mesh sieve, added with additional magnesium stearate, mixed evenly, and compressed into tablets.
[0027]
Embodiment 3
[0029] Bilastine 20% Mannitol 50.5% microcrystalline cellulose 20% Crospovidone 8% Povidone K30 0.5% Magnesium stearate 1% total 100%
[0030] Co-micronize the active ingredient Bilastine and part of mannitol in the prescription, and control the particle size of 90% of the volume or more particles to be in the range of 5-50 μm, so the excipients are passed through a 100-mesh sieve, and the micronized product Mix with the remaining lactose, microcrystalline cellulose, and crospovidone evenly in equal amounts, add an appropriate amount of PVP K30, make a soft material, squeeze and sieve through a 18-mesh sieve to granulate, and place the wet granules at a constant temperature of 50°C Dry for 2 hours, sieve the dry granules through a 24-mesh sieve, add additional magnesium stearate, mix well, and compress into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com