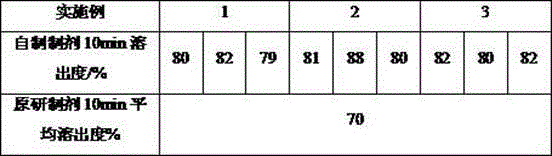
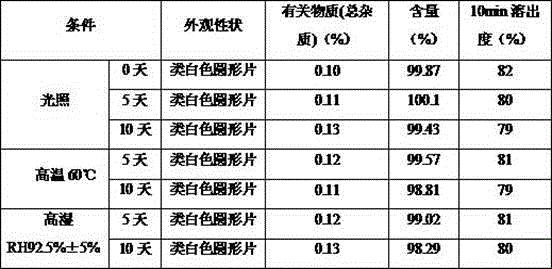
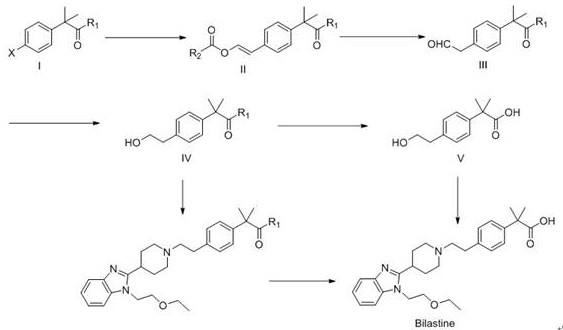
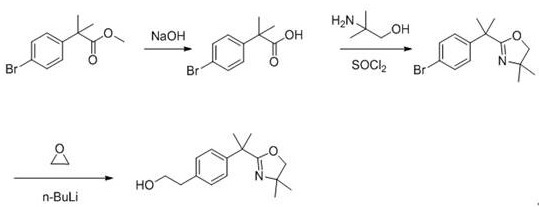
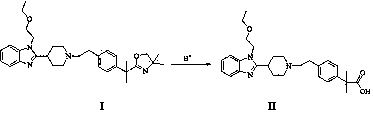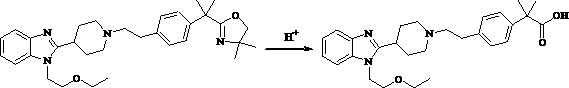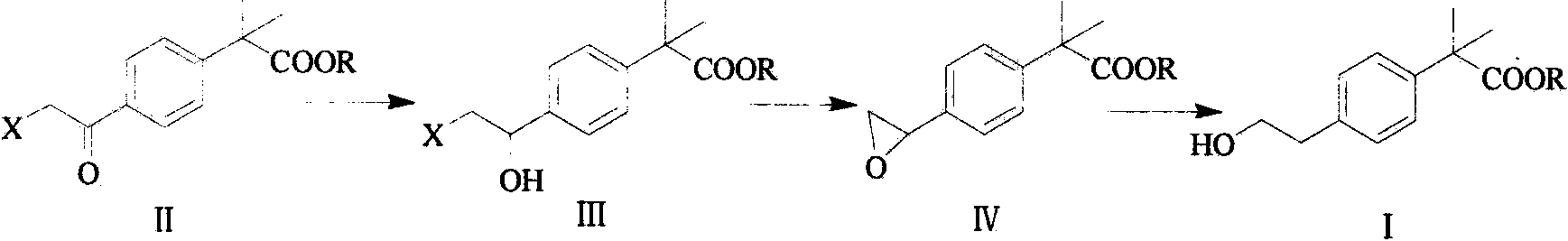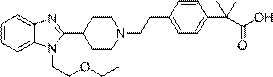Patents
Literature
54 results about "Bilastine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
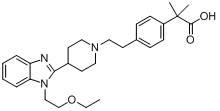
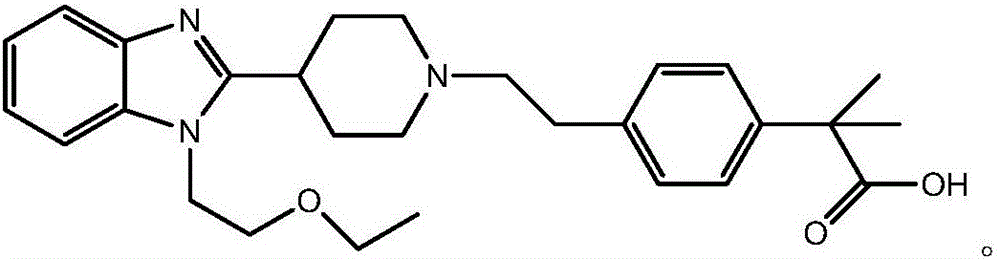
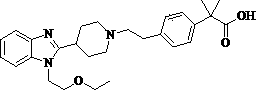
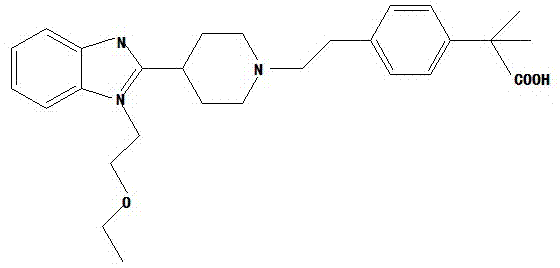
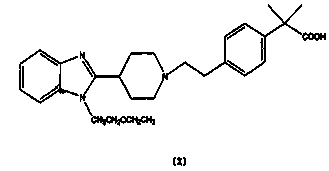
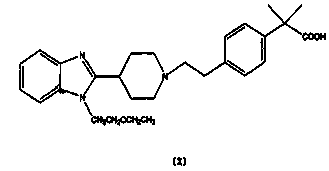
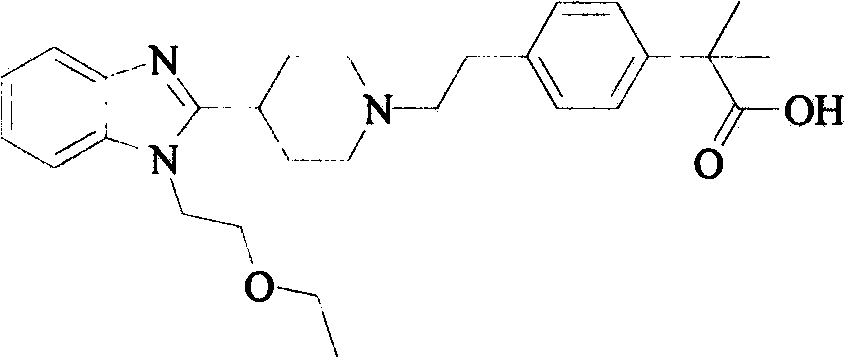
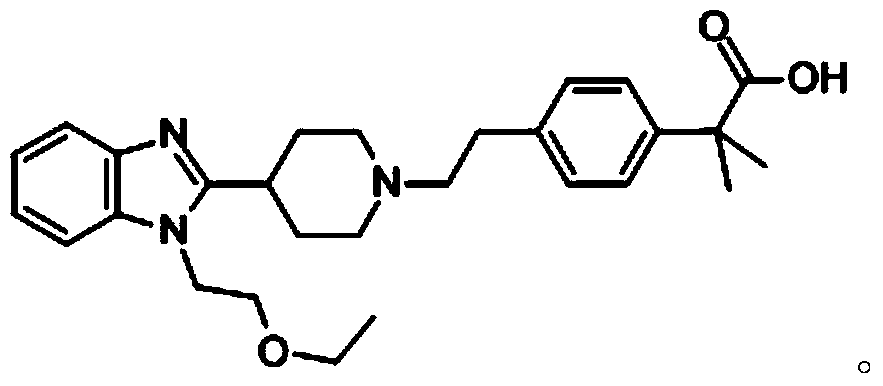
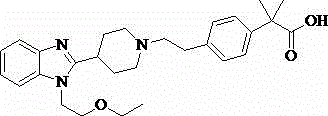
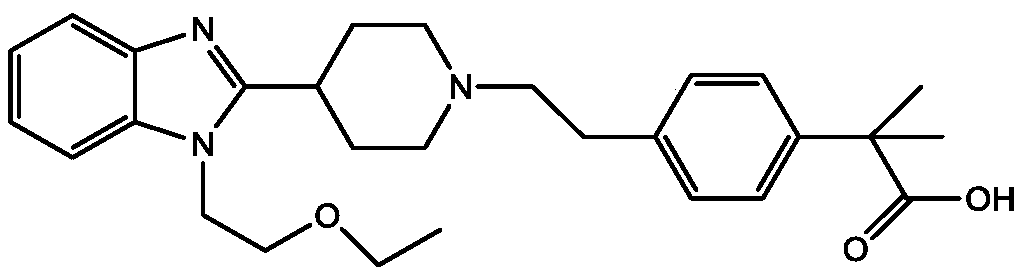
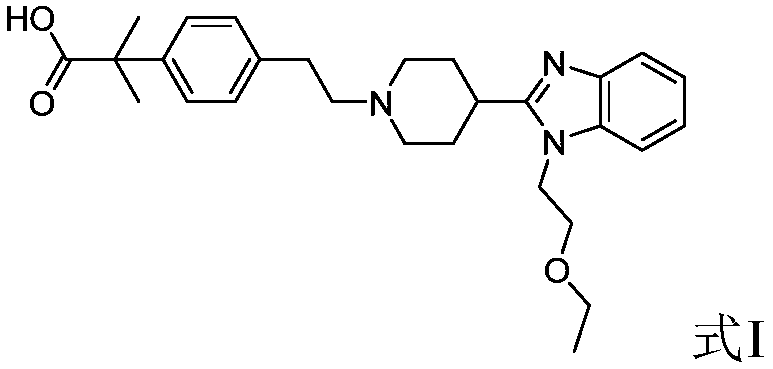
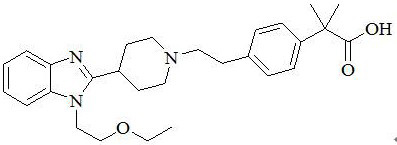
Bilastine, sold under the brand name Bilaxten among others, is a second-generation antihistamine medication which is used in the treatment of allergic rhinoconjunctivitis and urticaria (hives). It exerts its effect as a selective histamine H₁ receptor antagonist, and has an effectiveness similar to cetirizine, fexofenadine, and desloratadine. It was developed in Spain by FAES Farma.
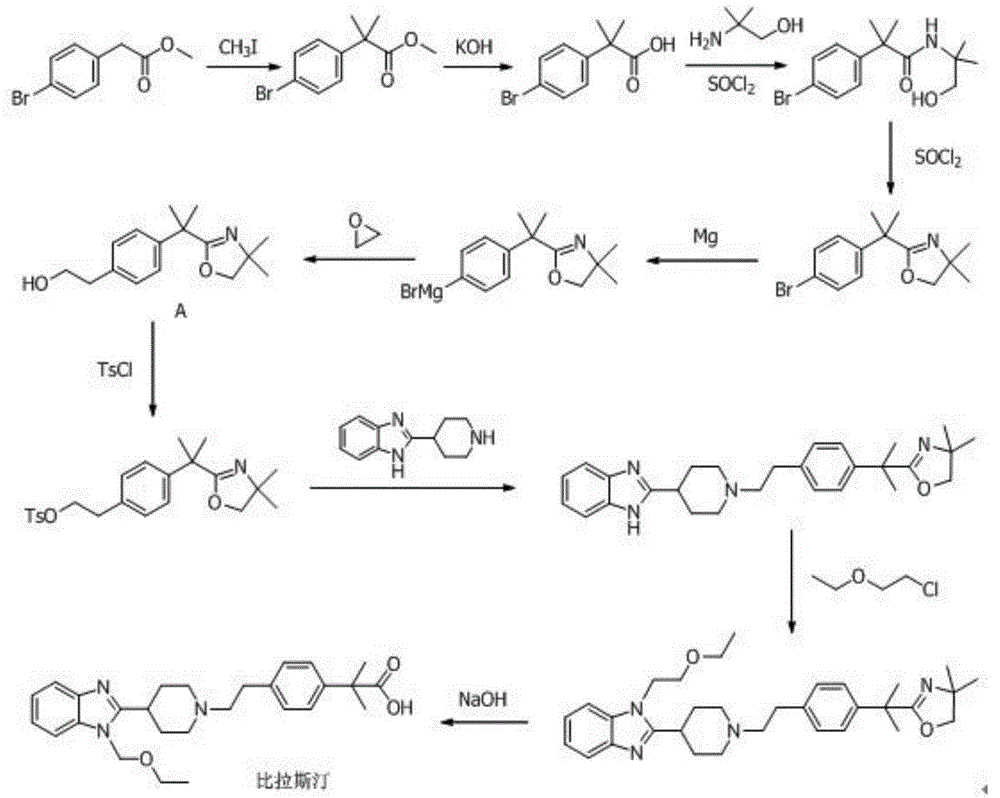
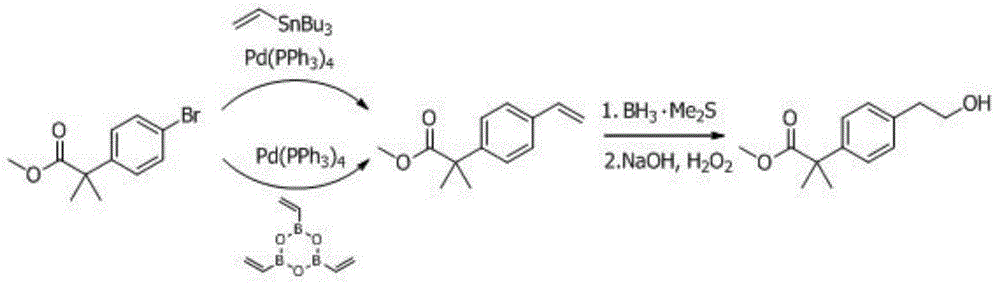
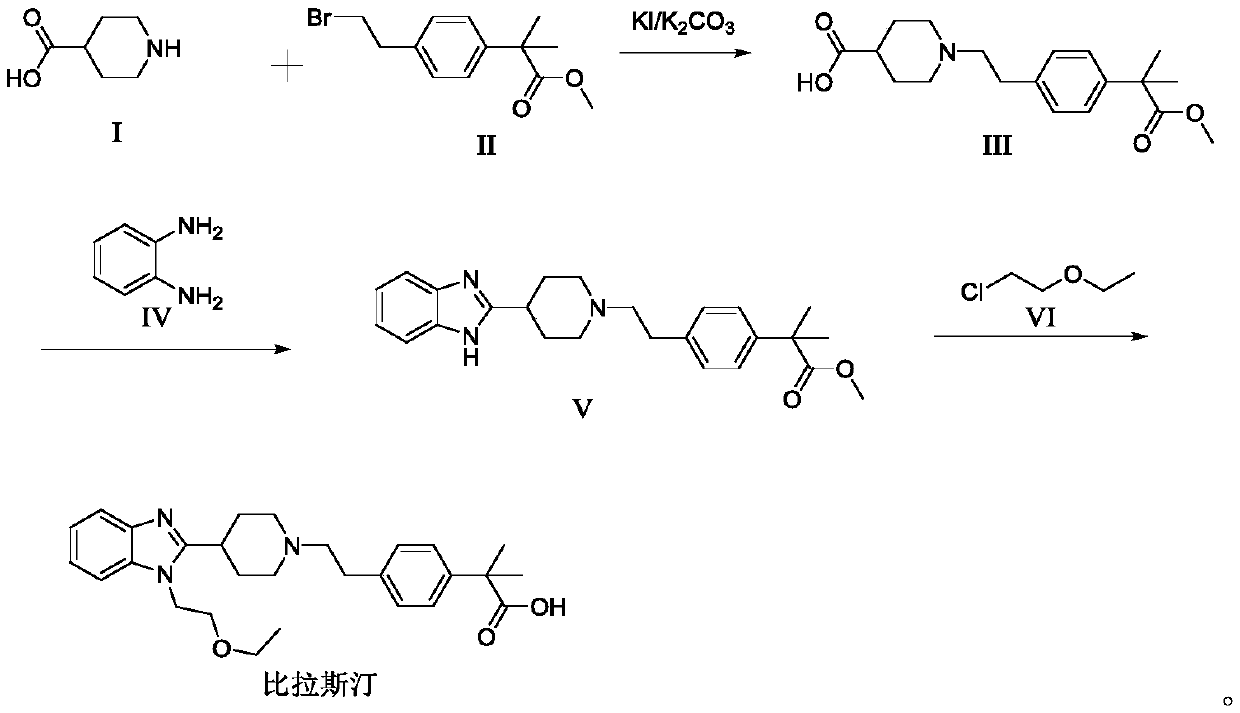
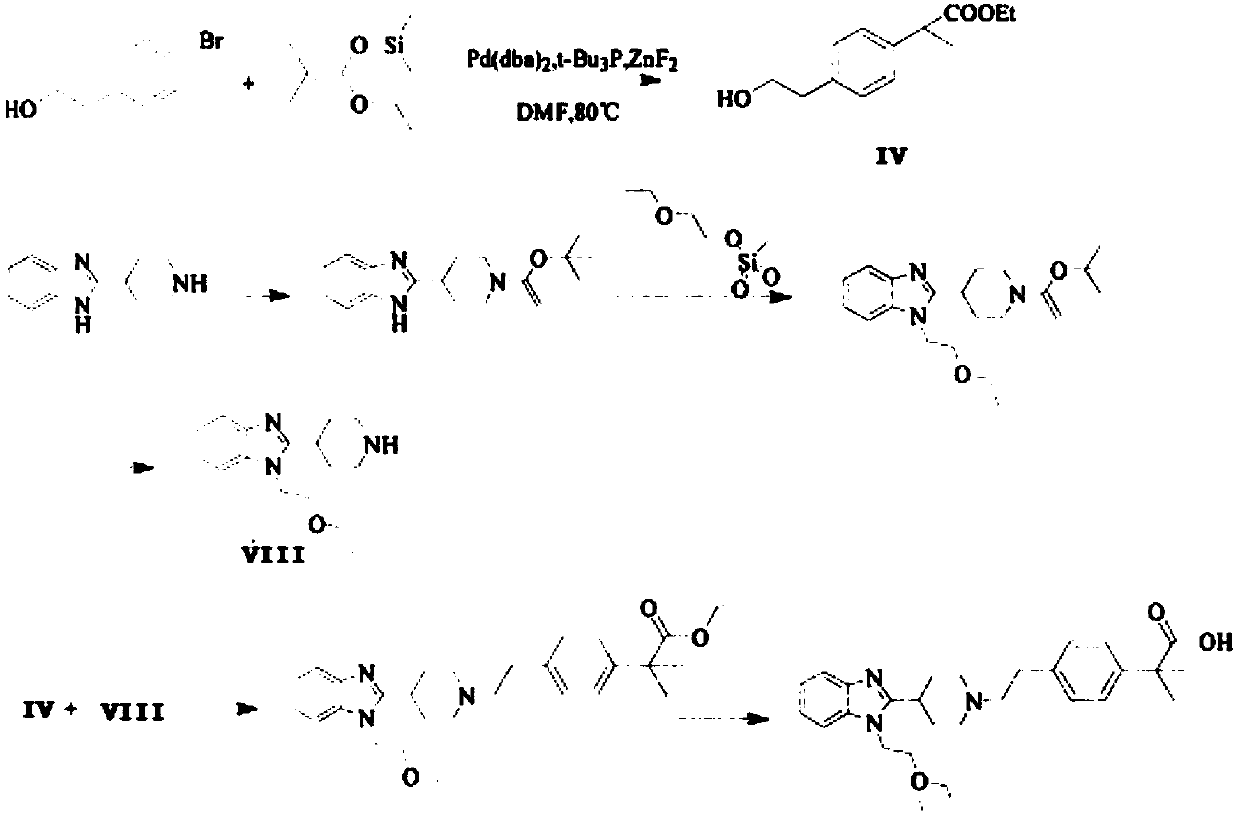
Preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and synthesis method of bilastine
InactiveCN102675101AAvoid expensive reagentsRaw materials are cheap and easy to getPreparation from carboxylic acid halidesPropanoic acidPtru catalyst
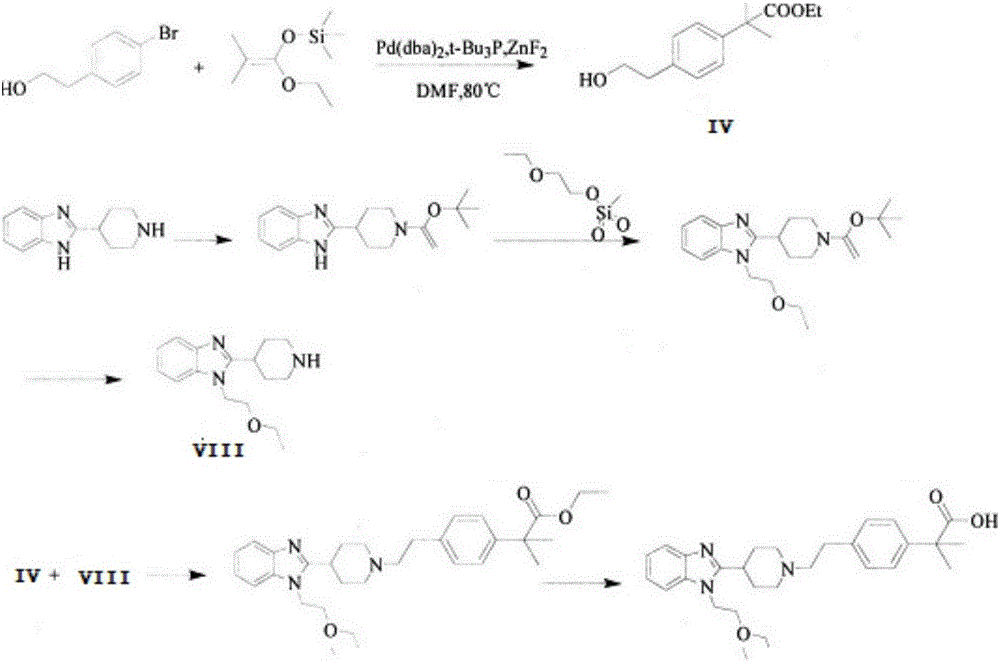
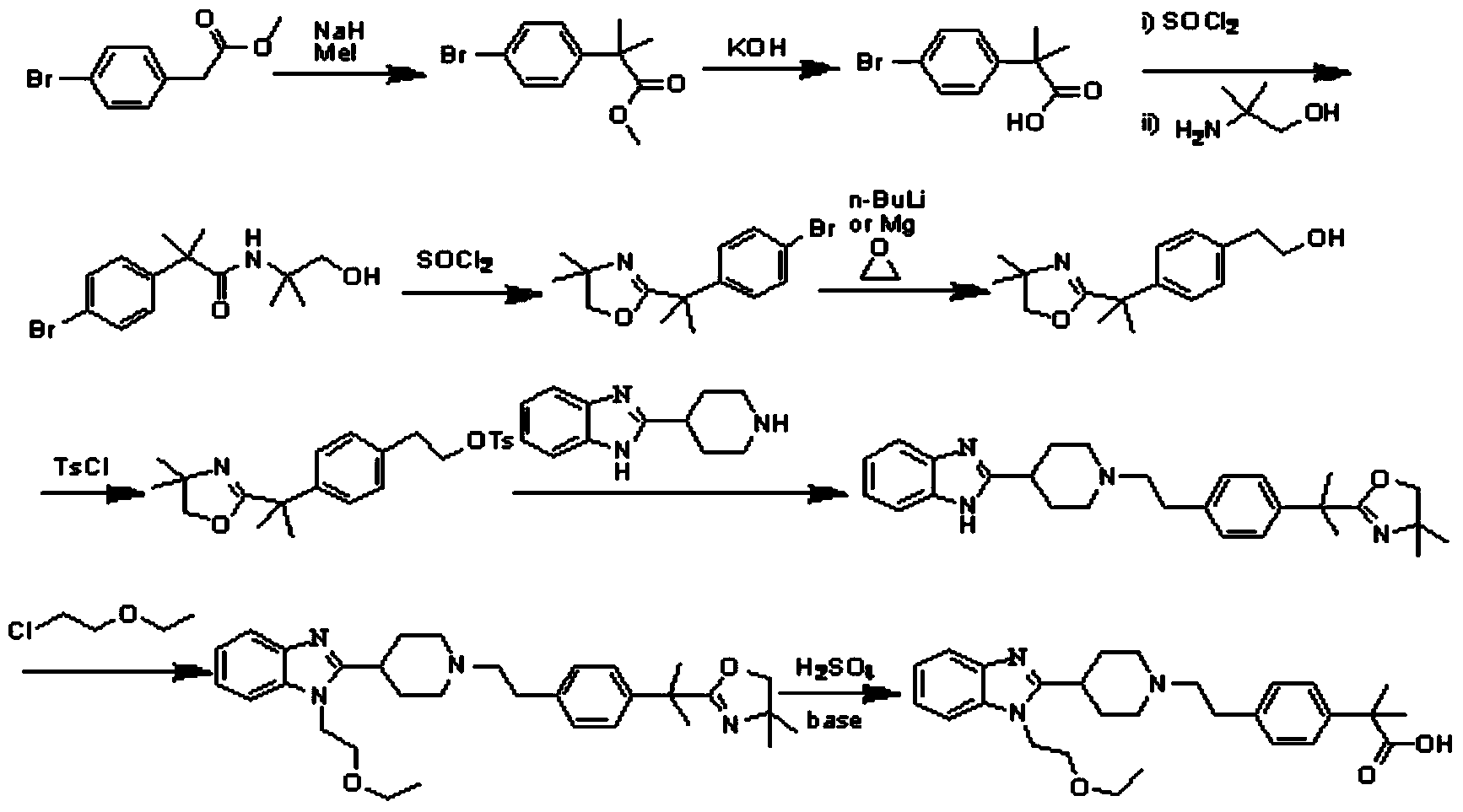
The invention discloses a preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and a synthesis method of bilastine, which comprises the following steps of: carrying out acylation reaction on 2, 2-dimethyl phenylacetate and halogen acetyl halides under the action of catalyst, to generate 2-(4-halogen acetyl) phenyl-2-methyl propionic ester; carrying out kishner-wolff-huang reduction reaction on the 2-(4-halogen acetyl) phenyl-2-methyl propionic ester, to reduce the carbonyl so as to generate the 2-(4-haloethyl) phenyl-2-methyl propionic ester; having condensation reaction with 1-ethoxy ethyl-2-pyridine-4-group benzimidazole by taking the 2-(4-haloethyl) phenyl-2-methyl propionic ester as a midbody to obtain esterified bilastine; and hydrolyzing, to generate the bilastine. The novel synthesis method of the bilastine provided by the invention can easily obtain raw materials, and is simple to operate, lower in cost, environment-friendly, and completely suitable for the industrial production.
Owner:王蕾
Bilastine preparation method
ActiveCN106146459ALower reaction costMild reaction conditionsOrganic compound preparationSulfonic acid esters preparationState of artAlkyl transfer
The invention discloses a Bilastine preparation method. The Bilastine preparation method includes that 2-nitroaniline which is low in price and easy to obtain is taken as a raw material which is subjected to reduction-n-cyclohexylmaleimide reaction, alkylation reaction, hydrolyzing and coupling prior to hydrolyzing to obtain Bilastine. With the method, shortcomings that harsh operation conditions, high toxicity, expensive raw materials and tedious operation in the prior art are overcome, reaction conditions in each step are moderate, and the synthetic method is simple in operation, easy to deal with, few in side products, high in yield and purity, low in production cost and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method of Bilastine
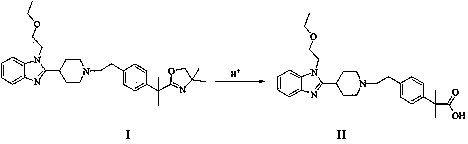
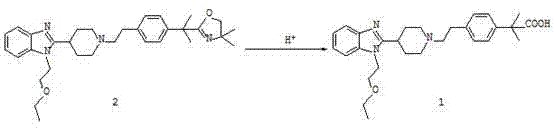
The invention belongs to the field of medical chemistry, and relates to a preparation method of Bilastine. The preparation method comprises following steps: adding compound 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazole-2-yl)-1-methyl-ethyl]-phenyl}-ethyl)-piperidine-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole into water containing organic acid, then subjecting the mixture to a thermal-reflux reaction for 1 to 36 hours, and finally obtaining Bilastine after post-processing. The preparation method has the advantages of mild reaction conditions, simple operation and easy industrialization.
Owner:BEIJING VENTUREPHARM BIOTECH
Bilastine crystal form and preparation method thereof
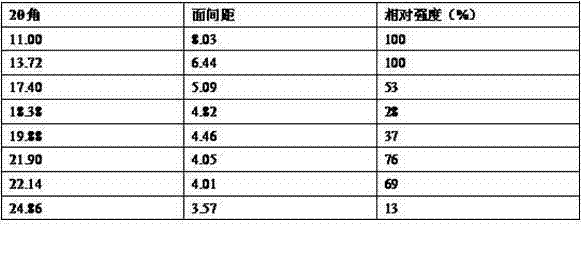
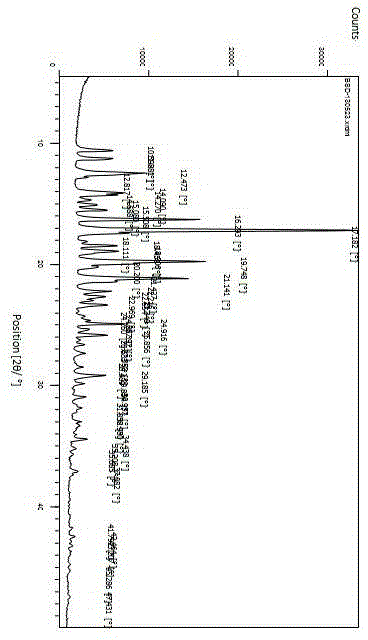
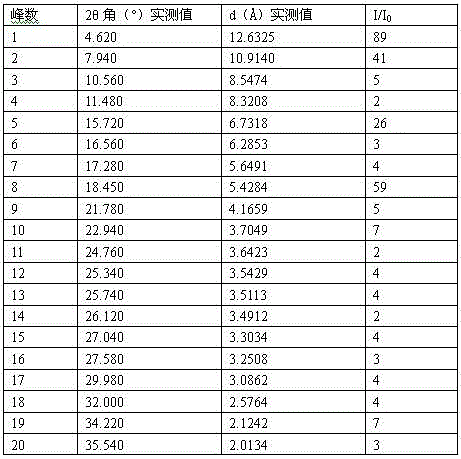
The invention relates to preparation of a medicine, and particularly relates to a crystal form of 2[4-(2-{4-[1-(2-ethoxyethyl)-1H-benzimidazole-2-yl]-piperidine-1-yl}-ethyl)-phenyl]-2-methyl propionic acid and a preparation method. The invention provides a stable bilastine crystal form and a preparation technology scheme thereof. The X-ray powder diffraction 2 theta (+ / -0.2) data of the stable bilastine crystal form are 11.30, 12.50, 17.18, 18.94, 19.80, 21.14, 22.68 and 24.92.
Owner:北京博泽德润医药科技开发有限公司
Bilastine orally disintegrating tablet and preparing method thereof
InactiveCN104398481AOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletLactose
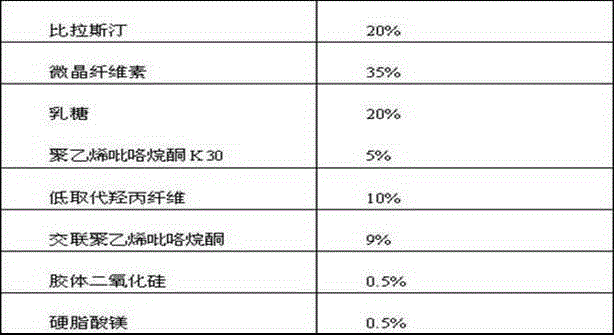
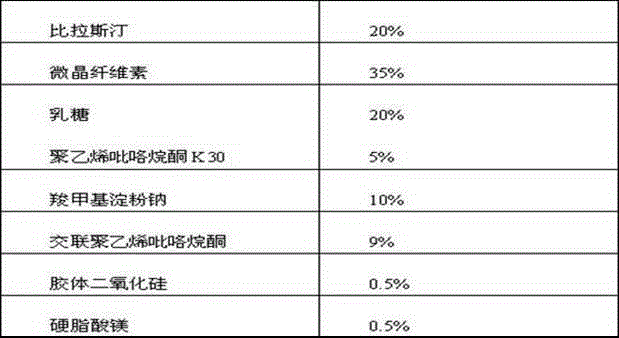
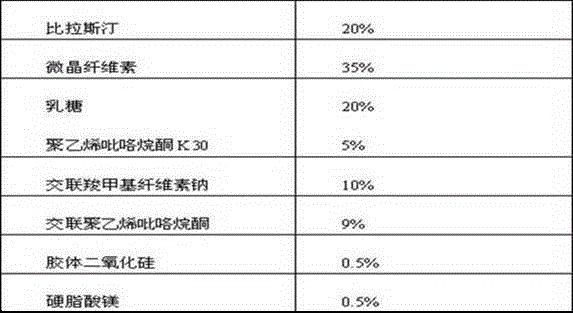
The invention belongs to the technical field of medicines, and relates to a bilastine orally disintegrating tablet and a preparing method of the bilastine orally disintegrating tablet. The bilastine orally disintegrating tablet comprises, by weight, 15%-30% of bilastine, 35%-74% of microcrystalline celluloses, 10%-40% of lactose, 0%-10% of dry binding agents, 2.0%-10% of disintegrating agents and 0.4%-3% of lubricating agents. According to a preparation process, the direct powder compression technology and the dry granulating technology are adopted. The bilastine orally disintegrating tablet is simple in preparation process, low in cost, convenient to take and high in effect taking on adaptation diseases. After being orally taken, the bilastine orally disintegrating tablet is rapidly disintegrated and dispersed into fine particles or powder in the oral cavity, and is particularly suitable for patients difficult in swallowing and psychopaths; in addition, before reaching the gastrointestinal tract, the preparations has been generated in the mode of fine particles or powder, the medicines are dissolved in an accelerated mode; the distribution area of the medicines in the gastrointestinal tract is large; the number of absorbing points is large; and the bioavailability of the bilastine orally disintegrating tablet can be improved.
Owner:万全万特制药江苏有限公司
Preparation method of bilastine
ActiveCN104177331ASimple preparation processSimple methodOrganic chemistryCombinatorial chemistryBilastine
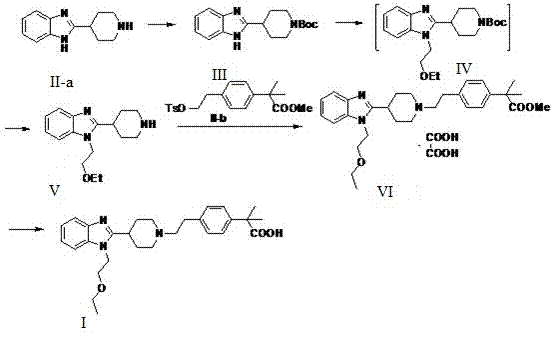
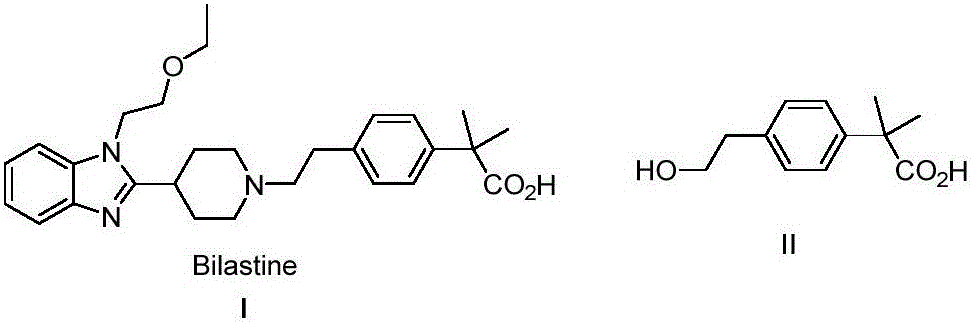
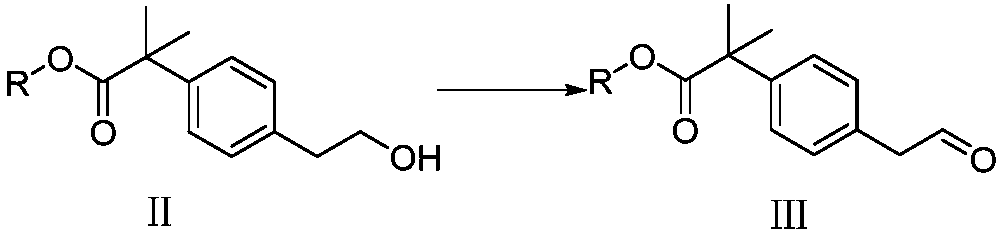
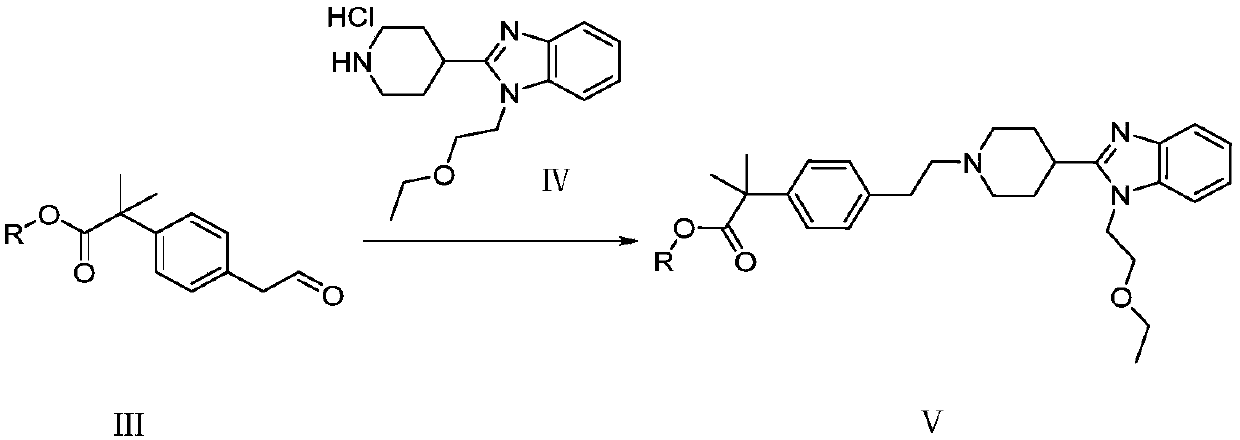
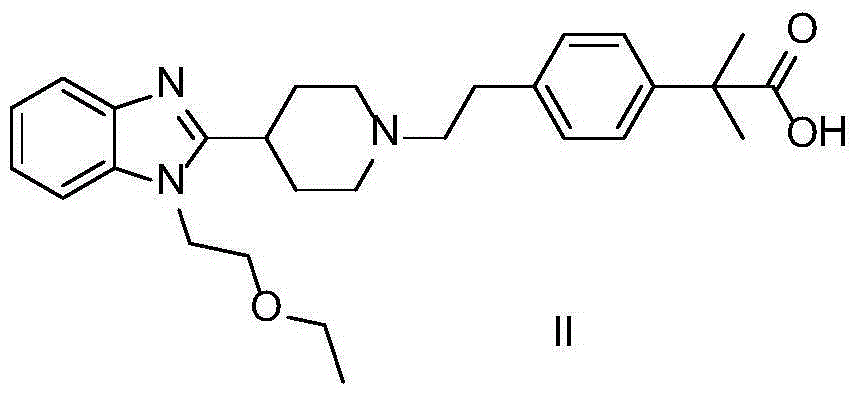
The invention provides a preparation method of bilastine. The preparation method of bilastine comprises the following steps: converting a compound with a structure shown as a formula (II) to a compound with a structure shown as a formula (III), and then hydrolyzing the compound with the structure shown as the formula (III) to obtain bilastine. A preparation method of the compound with the structure shown as the formula (II) is relatively simple; a method for converting the compound with the structure shown as the formula (II) to the compound with the structure shown as the formula (III) is also relatively simple; accordingly, the preparation method of bilastine is relatively low in cost and relatively high in total yield.
Owner:BEIJING COLLAB PHARMA
Method for preparing bilastine
The invention belongs to the field of medicine chemicals, and particularly relates to a method for preparing bilastine. The method comprises the following steps of dissolving 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazole-2-based)-1-methyl-ethyl]-phenyl}-ethyl)-piperidine-4-based]-1-(2-ethoxyl-ethyl)-1H-benzimidazole in an organic solvent, adding a certain amount of 4-butyl ammonium hydrogen sulfate and a hypochlorite water solution, reacting for 1-24 hours based on heating at a certain temperature, and treating the reaction liquid to obtain bilastine. The method is mild in conditions and simple in operation; and the method has the advantages of easily available raw materials, simplicity in operation, high yield and convenience for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Bilastine-containing pharmaceutical composition and preparation method thereof
InactiveCN103356616AGuaranteed bioavailabilityEasy to operateOrganic active ingredientsPharmaceutical non-active ingredientsWater solubleBioavailability
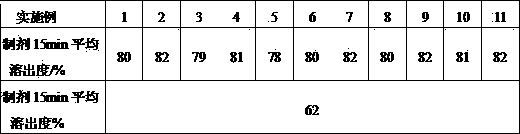
The invention belongs to the technical field of medicines, and relates to a bilastine pharmaceutical composition with good dissolution effect. The pharmaceutical composition comprises bilastine, a water-soluble excipient, a disintegrating agent, a lubricating agent and the like, wherein the bilastine is micronized, the grain size of 90% of the bilastine is controlled to 5 to 50 microns and is specifically preferable to 10 to 20 microns. A medicine prepared by the preparation method shows good dissolution effect, and therefore, the bioavailability of the medicine can be effectively improved.
Owner:BEIJING VENTUREPHARM BIOTECH
Bilastine compound and preparation method thereof
ActiveCN104530002AImprove stabilityHigh purityOrganic active ingredientsSenses disorderSolubilitySulfonyl chloride
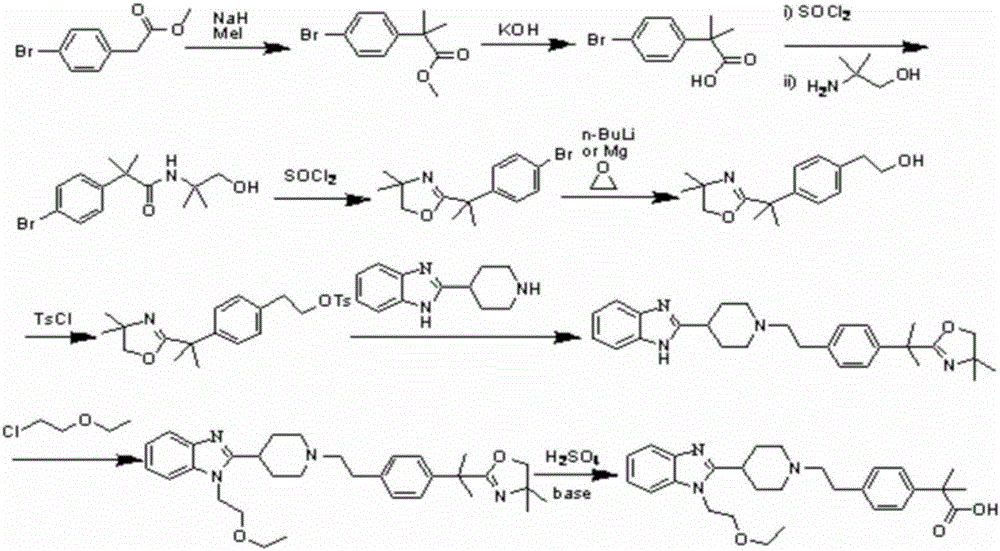
The invention belongs to the technical field of medicines, and specifically relates to a bilastine compound and a preparation method of the compound. The bilastine compound is high in stability and not obvious in moisture-absorption weight gain under a high-humidity condition, and related substances are not increased; compared with bilastine in other crystal forms, the bilastine compound is high in solubility and outstanding in physical and chemical performances. The preparation method comprises the following steps: by taking p-methyl phenethyl alcohol as a starting raw material, performing the sulfonylation reaction for hydroxy through sulfonyl chloride to obtain sulfonate; condensing sulfonate with 1-ethoxyethyl-2-piperidyl benzoglioxaline; performing bromination for benzyl; carrying out grignard reaction to introduce carboxyl to the benzyl, and then converting benzyl into ester; performing dimethylation for benzyl through iodomethane; finally hydrolyzing to obtain bilastine. The method involves seven synthesis reactions, and has the advantages that few synthesis steps are carried out, the reaction conditions are mild, the raw materials are easily obtained, the reaction process is simple, the yield is high, the cost is relatively low, the industrialization is easily carried out, and three-waste pollution is less.
Owner:天津梅花生物医药科技有限公司
Preparation method of bilastine key intermediate
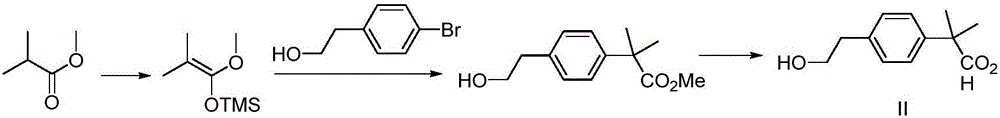
ActiveCN104276952AAvoid harsh conditionsRaw materials are easy to getOrganic compound preparationCarboxylic acid esters preparationMethyl propionateBilastine
The invention discloses a preparation method of a bilastine key intermediate 2-(4-ethoxy)-phenyl-2-methyl propionate. The method comprises the following steps: carrying out carbonyl reducing reaction on 2-(4-haloacetyl)-phenyl-2-methyl propionate under the action of a catalyst; carrying out cyclization in an alkaline solution; and then carrying out reduction reaction under the action of a catalyst, so as to obtain 2-(4-ethoxy)-phenyl-2-methyl propionate. According to the method disclosed by the invention, the severe condition in the prior art is avoided, and the method is available in raw materials, simple in operation, low in cost, friendly to environment and suitable for industrial production.
Owner:江苏华阳制药有限公司
Bilastine purifying method
The invention provides a method for refining and purifying 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazole-2-yl]-1-piperidine]ethyl]-alpha, alpha-dimethyl phenylacetic acid (Bilastine). Bilastine is used for treating allergic rhinitis and chronic idiopathic urticaria, has good safety and does not have the sedative effect and cardiac toxicity of common antihistamine drugs. In most of Bilastine preparation processes, the impurity content is high, and stubborn impurities are difficult to remove. The method for refining and purifying Bilastine, provided by the invention, comprises the steps of dissolving a crude product of Bilastine into a hot mixed solvent, cooling, precipitating, filtering, washing and drying to obtain high-purity Bilastine, wherein the impurity content meets the requirements.
Owner:万全万特制药江苏有限公司
Intranasal medicinal composition containing bilastine and mometasone furoate
InactiveCN103736098ARelieve bitternessRelieve irritationOrganic active ingredientsAerosol deliveryThaumatinMedicine
The invention provides an intranasal medicinal composition. The intranasal medicinal composition contains bilastine, mometasone furoate, and thaumatin serving as a bitterness and irritation alleviator.
Owner:于运红
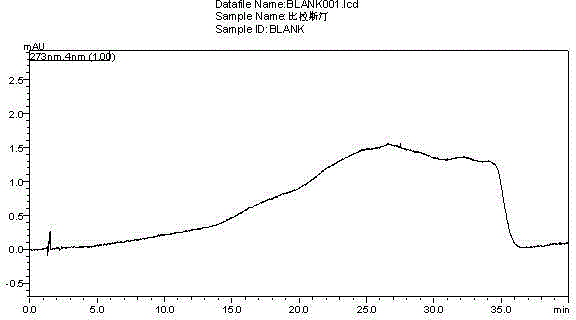
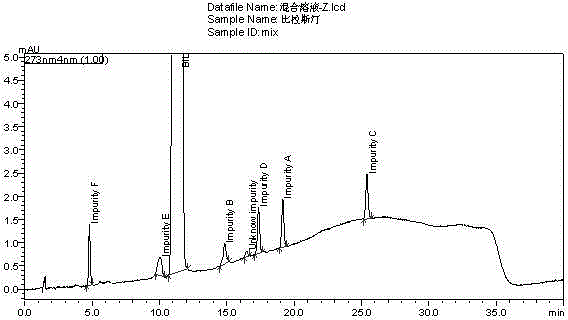
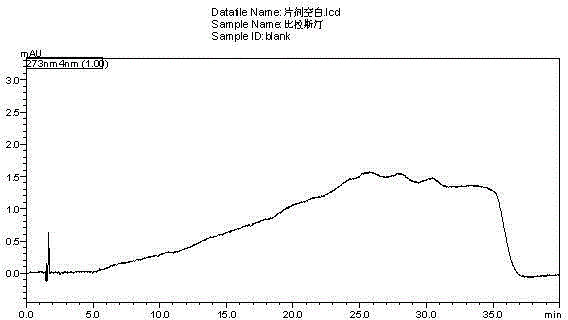
Method for separating and measuring bilastine and technical impurities in preparation of bilastine
ActiveCN105319288AQuality is easy to controlSolving Separation Assay ProblemsComponent separationSilanesGradient elution
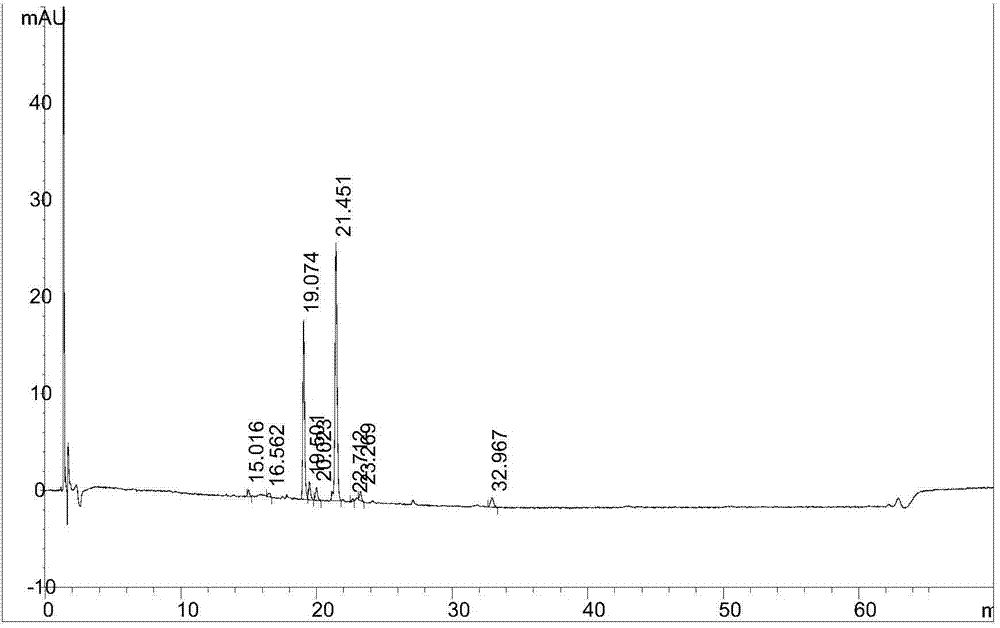
The invention belongs to the field of analytical chemistry, relates to a method for separating and measuring bilastine and technical impurities in a preparation of bilastine, and specifically relates to a method for separating and measuring bilastine and six technical impurities A-F (including a degradation product) in the preparation of bilastine by adopting high performance liquid chromatography. An adopted chromatographic column takes octylsilane bonded silicone as a filler, and adopts an inorganic salt buffer system with an ion-pairing agent added and an organic solvent, wherein the inorganic salt buffer system and the organic solvent is according to a certain ratio, as a moving phase for gradient elution. According to the method, the technical impurities A-F of bilastine and the degradation product can be totally separated. The method is simple to operate and good in reproducibility. The content of a raw medicine of bilastine and relevant materials in the preparation can be effectively measured, and the method is good in specificity.
Owner:CHONGQING HUAPONT PHARMA
Method for preparing bilastine
The invention relates to a method for preparing bilastine. The method comprises the following steps: adding a compound I oxazolol to water, adding a phase transfer catalyst, p-toluenesulfonyl chlorideand sodium hydroxide, stirring and reacting all above substances, and performing filtration to obtain oxazolol sulfonate; adding the sulfonate to water, adding 2-(4-piperidinyl)-1-H-benzimidazole andthe phase transfer catalyst, adding sodium carbonate or potassium carbonate, heating the obtained suspension, performing a reaction for 3-5 h, filtering the obtained intermediate II, adding the intermediate II to a strong polar aprotic solvent, adding sodium hydroxide and the phase transfer catalyst, adding ethylene glycol monoethyl ether tosylate, stirring and reacting the obtained mixture at -20-60 DEG C, filtering the obtained reaction product, and washing the filtered reaction product with water to obtain an intermediate III; and adding the intermediate III to an aqueous solution of an organic acid, performing refluxing for 3-5 h, adding water, adding a strong alkali until saturation, refluxing the obtained solution for 3-5 h to generate a bilastine salt insoluble in the saturated alkaline solution, and performing extraction to obtain the bilastine. The method has the advantages of mild reaction conditions, simplicity in operation, greenness, environmental protection, high yield,and convenience in industrial production.
Owner:湖北省医药工业研究院有限公司
Preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and synthesis method of bilastine
InactiveCN102675101BAvoid expensive reagentsRaw materials are cheap and easy to getPreparation from carboxylic acid halidesPropanoic acidPtru catalyst
The invention discloses a preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and a synthesis method of bilastine, which comprises the following steps of: carrying out acylation reaction on 2, 2-dimethyl phenylacetate and halogen acetyl halides under the action of catalyst, to generate 2-(4-halogen acetyl) phenyl-2-methyl propionic ester; carrying out kishner-wolff-huang reduction reaction on the 2-(4-halogen acetyl) phenyl-2-methyl propionic ester, to reduce the carbonyl so as to generate the 2-(4-haloethyl) phenyl-2-methyl propionic ester; having condensation reaction with 1-ethoxy ethyl-2-pyridine-4-group benzimidazole by taking the 2-(4-haloethyl) phenyl-2-methyl propionic ester as a midbody to obtain esterified bilastine; and hydrolyzing, to generate the bilastine. The novel synthesis method of the bilastine provided by the invention can easily obtain raw materials, and is simple to operate, lower in cost, environment-friendly, and completely suitable for the industrial production.
Owner:王蕾
Method for preparing novel crystal form of bilastine
InactiveCN104151290AOrganic active ingredientsOrganic chemistry methodsOrganic solventPhysical chemistry
The invention relates to a method for preparing a novel crystal form of bilastine. The method comprises the following steps: dissolving bilastine in a mixed solvent of an organic solvent and water, heating until all bilastine is dissolved, and cooling and crystallizing to obtain the novel crystal form of bilastine.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation method of new anti-allergic medicine Bilastine intermediate
ActiveCN106565467AMild reaction conditionsSimple and fast operationGroup 4/14 element organic compoundsPreparation from nitrilesMetal catalystRoom temperature
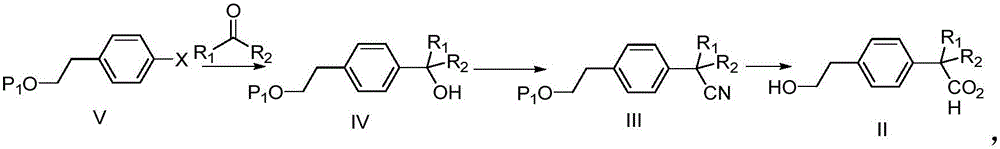
The invention discloses a preparation method of a new anti-allergic medicine Bilastine intermediate. Quaternary carbon atoms difficult to construct are constructed by using a mild addition reaction, carboxylic groups are introduced at room temperature by using a very cheap and easily available raw material, and the unique two-phase property of carboxylic acid is used to purify, so an expensive metal catalyst is not needed in the synthesis process, and strict enforcement conditions are avoided, thereby the preparation method of the Bilastine intermediate has the advantages of mild reaction conditions, simplicity in operation, low synthesis cost, and suitableness for large-scale production.
Owner:杭州励德生物科技有限公司
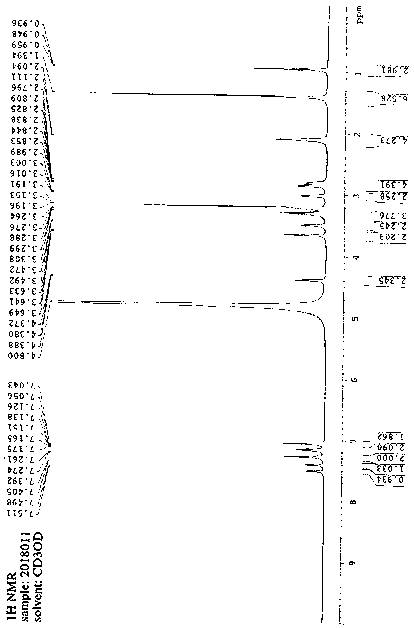
Bilastine compound
Belonging to the medical technology field, the invention in particular relates to a Bilastine compound and a preparation method thereof. The Bilastine obtained by the invention has the advantages of: chemical purity of 99.9%, maximum impurity of less than 1 per thousand, optical purity up to 99.96 percent enantiomeric excess, and good stability. The compound provided by the invention has the characteristics of low production cost and stable quality, and is suitable for industrialized production.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Bilastine detection method
InactiveCN104730194AEasy to separateEfficient separationComponent separationInorganic saltsOrganic solvent
The invention provides a Bilastine detection method. The Bilastine detection method comprises the steps of performing chromatographic analysis on a to-be-detected test of Bilastine, and obtaining each impurity in Bilastine, wherein a chromatographic analysis mobile phase is an inorganic salt buffer solution containing an ion pair reagent and an organic solvent. By adopting the inorganic salt buffer solution containing the ion pair reagent to serve as the chromatographic analysis mobile phase, the detection method can well separate each impurity in Bilastine and improve detection accuracy and can be used for quality monitoring of Bilastine raw material and preparations of the Bilastine raw material.
Owner:BEIJING COLLAB PHARMA
Preparation method of bilastine
ActiveCN110903278AEasy to routeRaw materials are easy to getOrganic chemistryBulk chemical productionPhenylacetic acidEthyl group
The invention belongs to the technical field of medicines, and discloses a preparation method of bilastine. 4-piperidinecarboxylic acid and methyl alpha,alpha-dimethyl-4-(2-bromoethyl)phenylacetate which are used as raw materials to prepare methyl alpha,alpha-dimethyl-4-[2-[4-formylpiperidyl]ethyl]phenylacetate, the methyl alpha,alpha-dimethyl-4-[2-[4-formylpiperidyl]ethyl]phenylacetate reacts with o-phenylenediamine to generate methyl alpha,alpha-dimethyl-4-[2-[4-[1H-2-benzimidazolyl]piperidin-1-yl]ethyl]phenylacetate, and chloroethyl ether is added into the obtained product to generate bilastine. The method for synthesizing bilastine by a three-step reaction has the advantages of simple route, easily available raw materials, mild reaction conditions, easiness in control, and suitablenessfor industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD +2
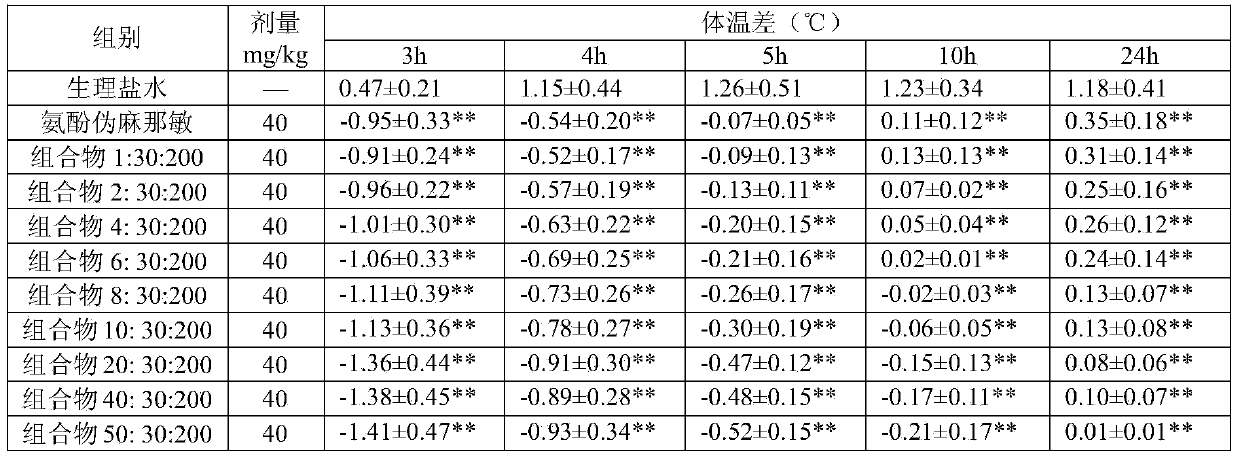
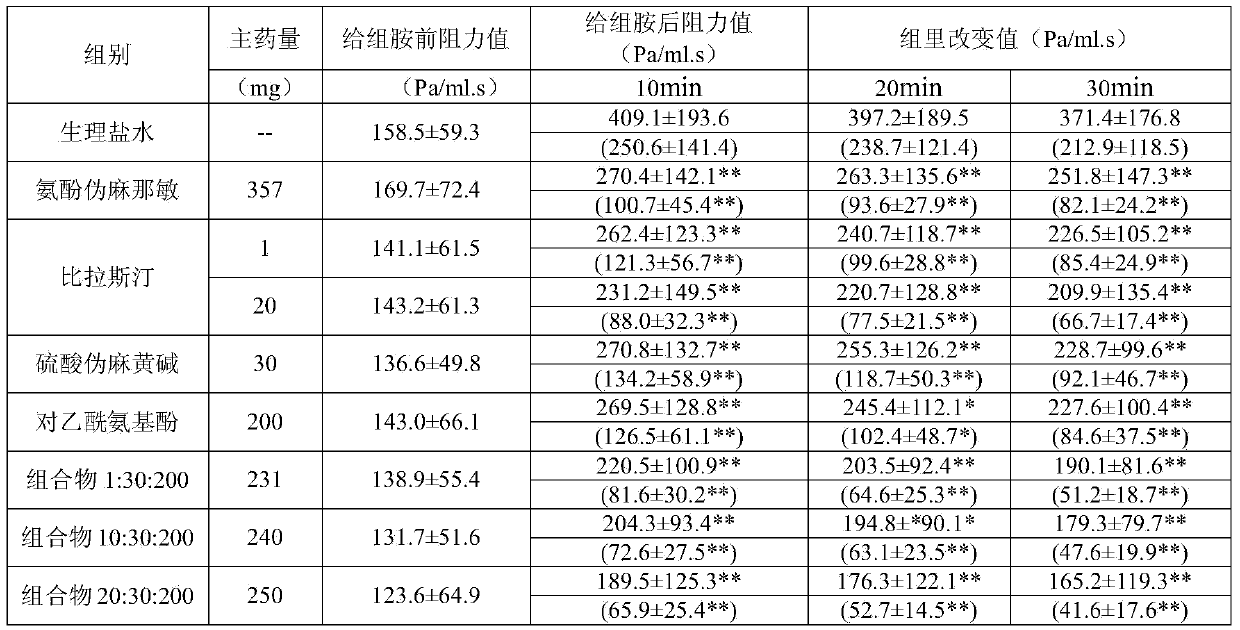
Compound cold medicinal composition containing bilastine
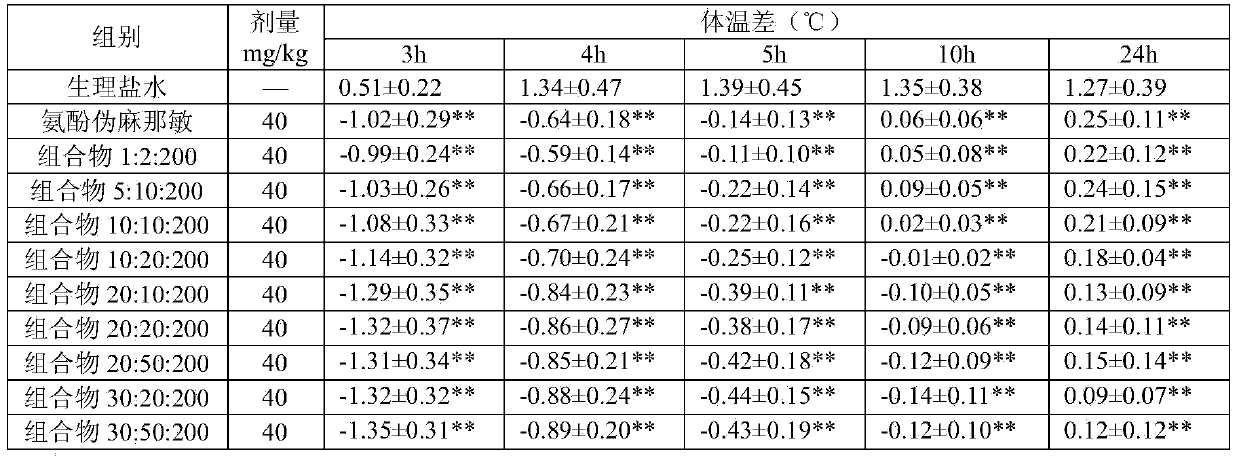
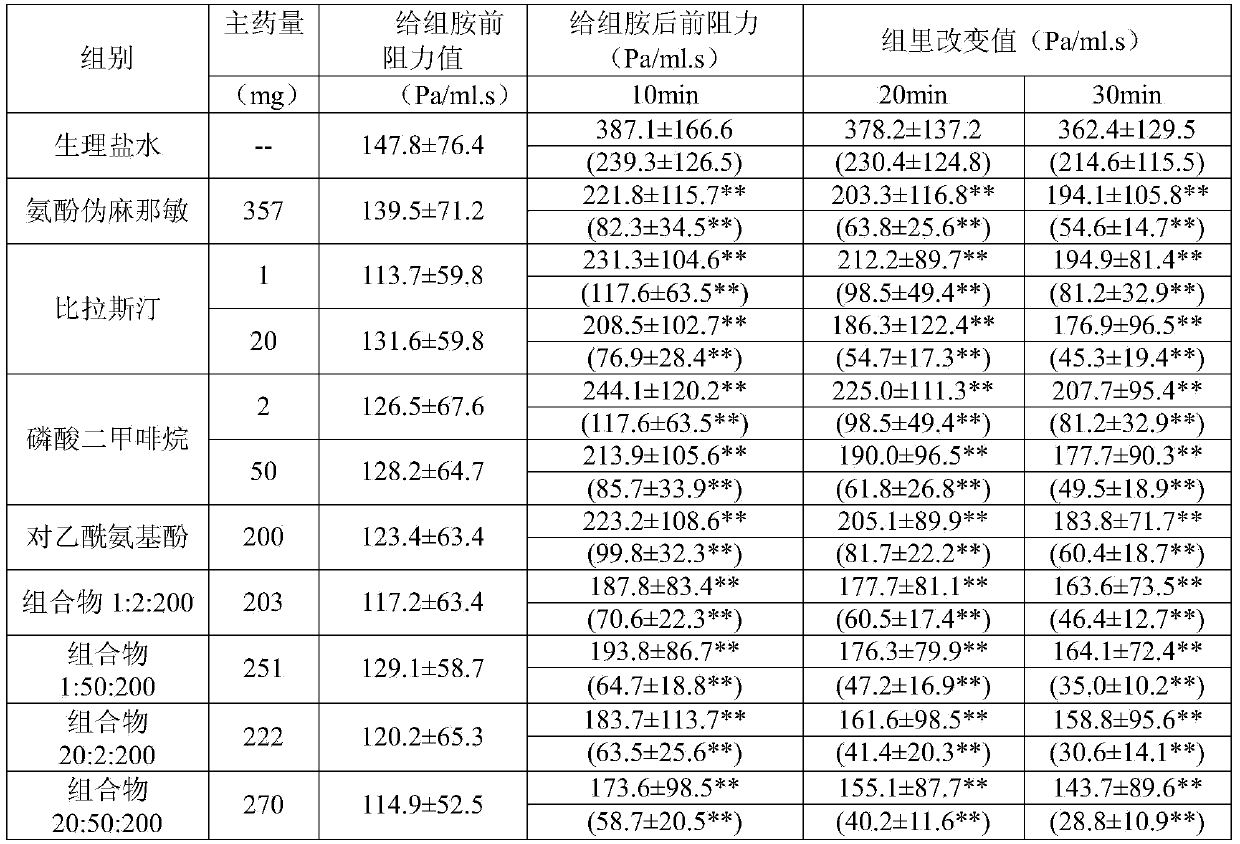
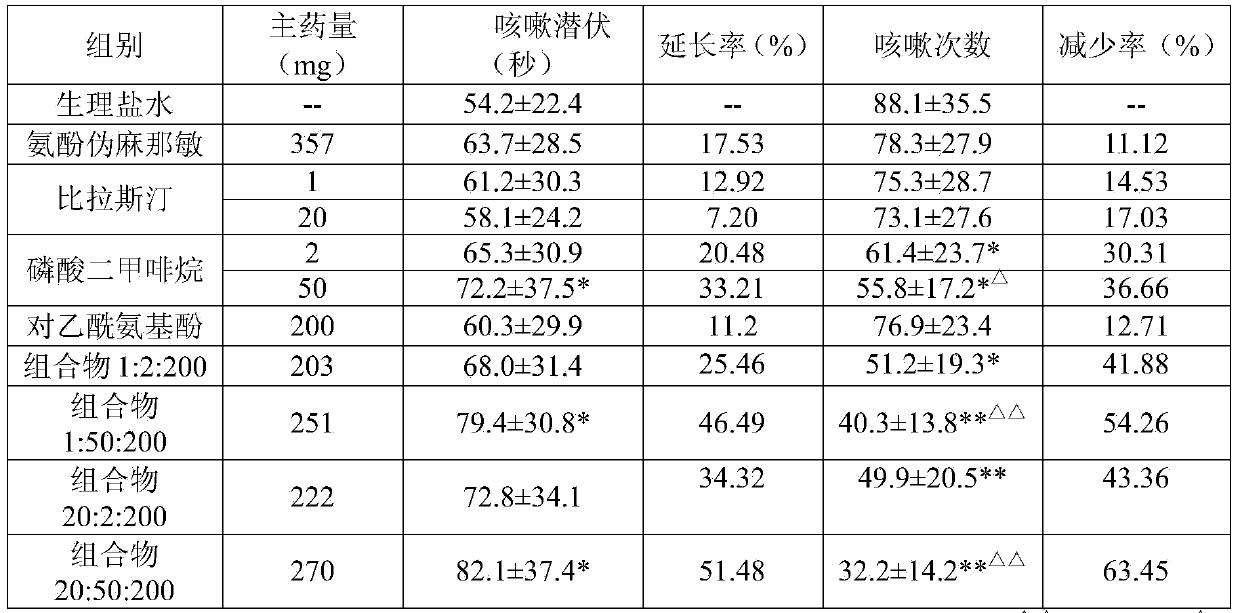
The invention relates to the field of medicine, and in particular relates to a compound cold medicinal composition containing bilastine. The compound medicinal composition contains three types of effective components, namely, bilastine, pseudoephedrine sulfate and acetaminophen. As proved by pharmacodynamic test, the compound medicinal composition has a synergistic effect when treating cold, and can be used for treating cold in clinic.
Owner:HEFEI IND PHARMA INST CO LTD
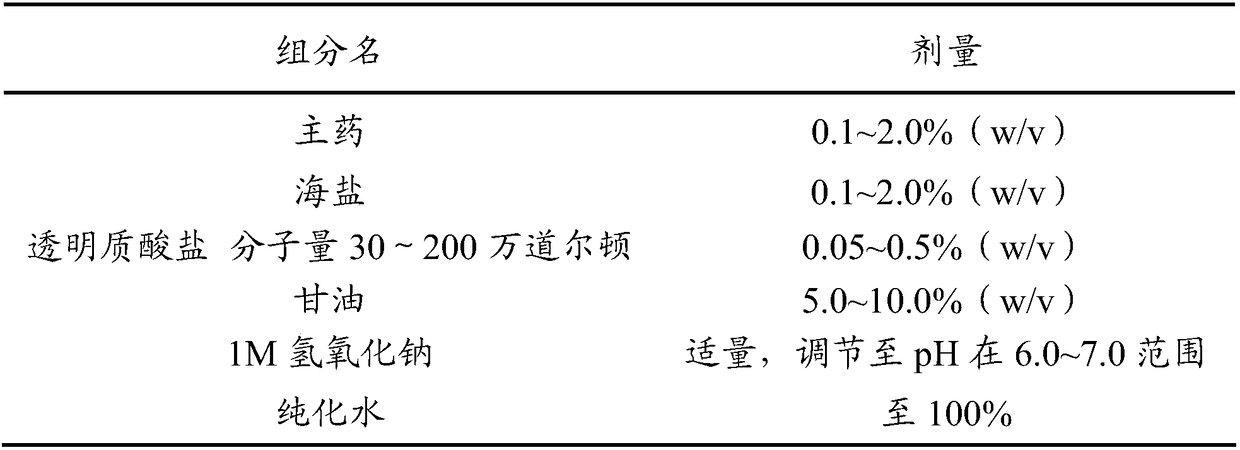
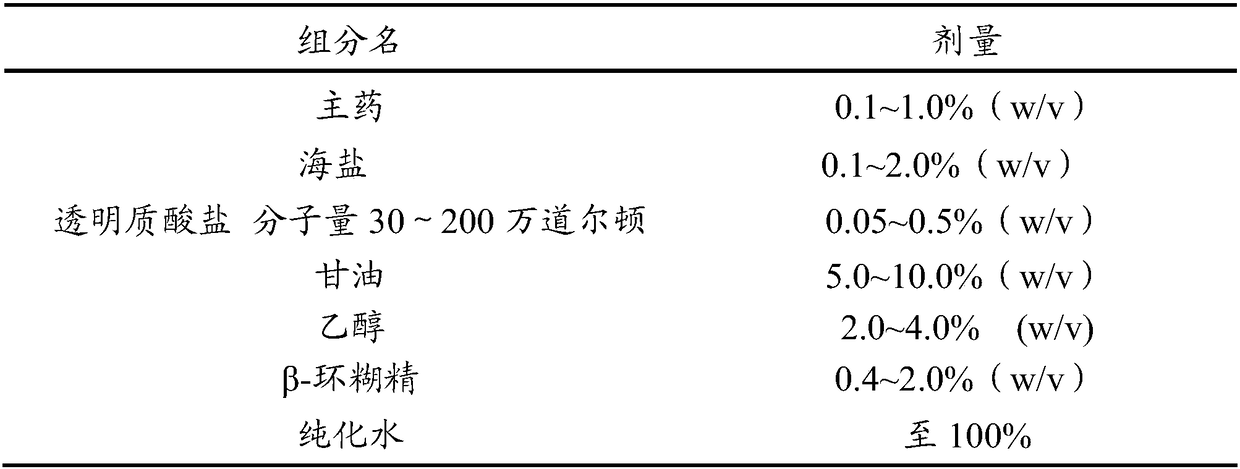
Antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof
InactiveCN109276715AUse lessImprove comfortPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIrritationGlycerol
The invention provides an antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof. The composition has high moisture retention and no irritation to a nasal mucosa. The composition comprises levocetirizine, azatadine, loratadine, ebastine, setastine, bilastine, clemastine, mizolastine, epinastine and moxifloxacin which are antiallergic medicine as main medicine, wherein the main medicine can be salts or free alkalis, sea salt as an osmotic pressure regulator, and a mixture of sodium hyaluronate and glycerin of different molecular weights as a moisturizing excipient. Plant essential oil can be contained in the composition. Preparations can be an aqueous solution, a cyclodextrin-coated suspension or a nanosuspension. Dosage forms include spray, aerosol and nose drops. Main indications include nasal dryness, runny nose, nasal itching, nasal obstruction and the like due to allergic rhinitis.
Owner:XIAN LIBANG PHARMA TECH
Bilastine tablet and preparation method thereof
InactiveCN110787140AProduct quality is easy to controlSuitable for mass productionOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical formulationTableting
The invention belongs to the technical field of medicinal preparations, and particularly relates to a bilastine tablet capable of realizing direct powder compression adopting silicified microcrystalline cellulose, and a preparation method thereof. The tablet is prepared from the following components by weight percent: 1 to 10 percent of bilastine, 10 to 90 percent of silicified microcrystalline cellulose, 3 to 20 percent of a disintegrating agent, 0.5 to 5 percent of a lubricating agent, and 0 to 5 percent of a flow aid. According to the bilastine tablet and the preparation method thereof provided by the invention, the defects of poor fluidity and compressibility of the bilastine are overcome, a recipe and a technology of direct bilastine compression are realized, the preparation technology is simpler, the stability of the product quality is ensured, and the method is suitable for mass production.
Owner:BEIJING VENTUREPHARM BIOTECH
Treatment method of bilastine reaction mother liquor
InactiveCN103755683AEasy to separateEasy to operateOrganic chemistryAcid waterPharmaceutical Substances
The invention belongs to the field of pharmaceutical chemistry and relates to a treatment method of a bilastine reaction mother liquor. The treatment method is characterized in that the bilastine reaction mother liquor is dissolved in acid water after being spin-dried, and then stirred for 0-78 hours at a certain temperature, and finally, after-treatment refining is carried out to obtain the qualified product of bilastine, namely, a compound 1.
Owner:万全万特制药江苏有限公司
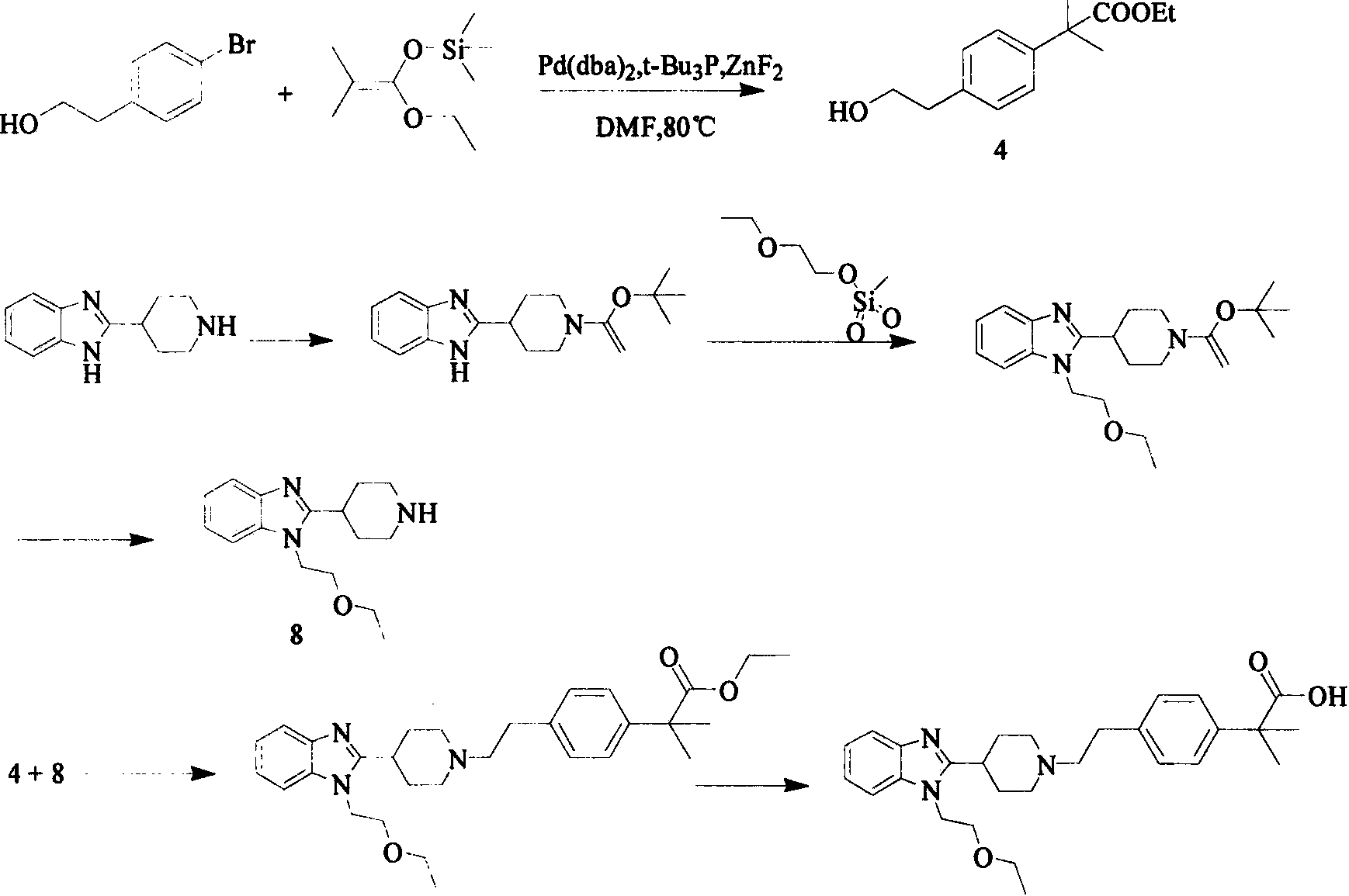
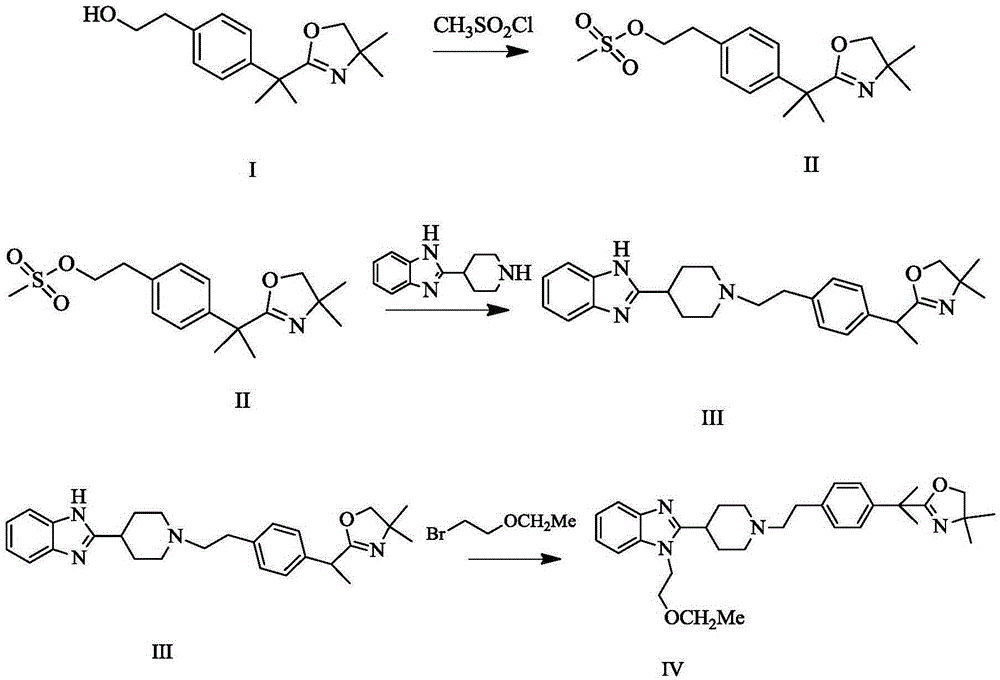
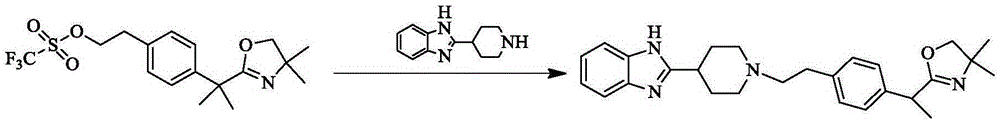
Bilastine intermediate preparation method
The present invention relates to a Bilasiting intermediate 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-2H-oxazol-2-yl)-1-methyl-ethyl]-phenyl}-ethyl)-piperidin-4-yl]-1H-benzimidazole preparation method, the compound 2-{1-[4-(2-hydroxyethyl) phenyl]-1-methylethyl}-4,5-2H-4,4-dimethyl-oxazole is dissolved in an organic solvent, trifluoromethanesulfonyl chloride is dissolved, and added dropwise to the system, after the completion of the addition, triethylamine is added for heating and refluxing for 1h to obtain structure II compound 2-[4-(1-(4,4-dimethyl-2H-oxazol-2-yl)-1-methylethyl) phenyl] ethyl triflate, and the resulting compound 2-[4-(1-(4,4-dimethyl-2H-oxazol-2-yl)-1-methylethyl) phenyl] ethyl triflate is dissolved in an organic solvent, under nitrogen protection, sodium methoxide is added for reaction for 2 h, 2-(4-piperidine)-1 H-benzimidazole is added for heating and refluxing for 2h to obtain 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-2H-oxazol-2-yl)-1-methyl-ethyl]-phenyl}-ethyl)-piperidin-4-yl]-1H-benzimidazole.
Owner:万全万特制药江苏有限公司
Preparation method of bilastine
The invention provides a preparation method of bilastine. Specifically the method comprises the following steps: oxidizing 4-hydroxyethyl phenyl methyl tert-butyrate to obtain 4-acetaldehyde phenyl methyl tert-butyrate, and carrying out a reductive amination reaction on the 4-acetaldehyde phenyl methyl tert-butyrate and a compound represented by a formula IV to obtain 4-[2-[1-(2-ethoxyethyl)benzimidazolyl]piperidyl]ethyl-phenyl methyl tert-butyrate (represented by a formula V); and hydrolyzing and acidifying the compound represented by the formula V to obtain bilastine. The method is high in yield, simple to operate and suitable for industrial production.
Owner:上海天慈中商药业有限公司
Bilastine-containing pharmaceutical composition and preparing method thereof
InactiveCN104398512ADisintegrates quicklyImprove dissolution efficiencyOrganic active ingredientsPill deliveryCellulosePharmaceutical drug
The invention belongs to the medical technical field, and relates to a bilastine pharmaceutical composition and a preparing method thereof; the preparaiton contains silica, bilastine, a cellulose filling agent, a disintegrant and a lubricant. The invention provides the bilastine oral solid preparation prepared by a non-wet granulation process; the process is simple to operate and suitable for industrialized production; and moreover, the prepared preparation is rapid to disintegrate and high in dissolution efficiency, and improves the bioavailability.
Owner:万全万特制药江苏有限公司
Preparation method of bilastine intermediate
PendingCN112110811AEasy to operateHigh purityOrganic compound preparationCarboxylic acid amides preparationPropanoic acidAcid derivative
The invention belongs to the field of medicinal chemistry, and relates to a preparation method of a bilastine intermediate 2-(4-(2-hydroxyethyl) phenyl)-2-methyl propionic acid and derivatives thereof. The 2-(4-(2-hydroxyethyl) phenyl)-2-methyl propionic acid and the derivative thereof are obtained by taking a 4-halogenated phenyl-2-methyl propionic acid derivative as a raw material through the steps of alkylation, hydrolysis, reduction and the like, and the compound can be used for synthesizing bilastine.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation method of bilastine oxide impurity
The invention discloses a preparation method of a bilastine oxide impurity. The preparation method comprises that bilastine undergoes a reaction in an alcohol solvent in the presence of an oxidizing agent to produce a bilastine oxide impurity in a high yield. The bilastine oxide impurity can be used for quality control of a bilastine bulk drug or its preparation and can be used as an impurity contrast.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Compound medicine composition containing bilastine and dimemorfan phosphate
InactiveCN104000825AAvoid adverse reactionsSignificant effectOrganic active ingredientsAntipyreticTraditional medicineBilastine
The invention relates to the field of medicines, and in particular relates to a compound medicine composition containing bilastine, dimemorfan phosphate and paracetamol, and a preparation method of the medicine composition. The pharmacodynamic tests show that the three components take a synergistic effect into play for preventing cold, and thus the medicine composition can be applied to clinical treatment of cold.
Owner:HEFEI IND PHARMA INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com