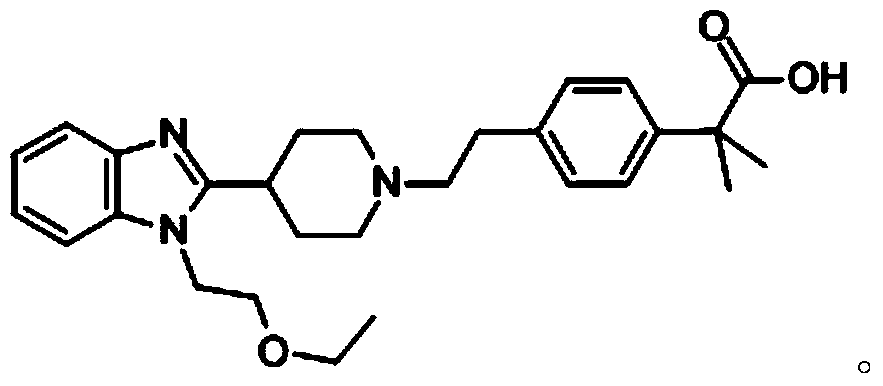
Intranasal medicinal composition containing bilastine and mometasone furoate
A technology of mometasone furoate and bilastine, which is applied in the directions of drug combinations, medical preparations containing active ingredients, non-active ingredients of polymer compounds, etc., can solve the problems of bilastine drops that are not reported in the literature. , to achieve the effect of improving medication compliance, excellent nasal sensation, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0034] Embodiment 1 and 2: Suspension for nasal cavity spray
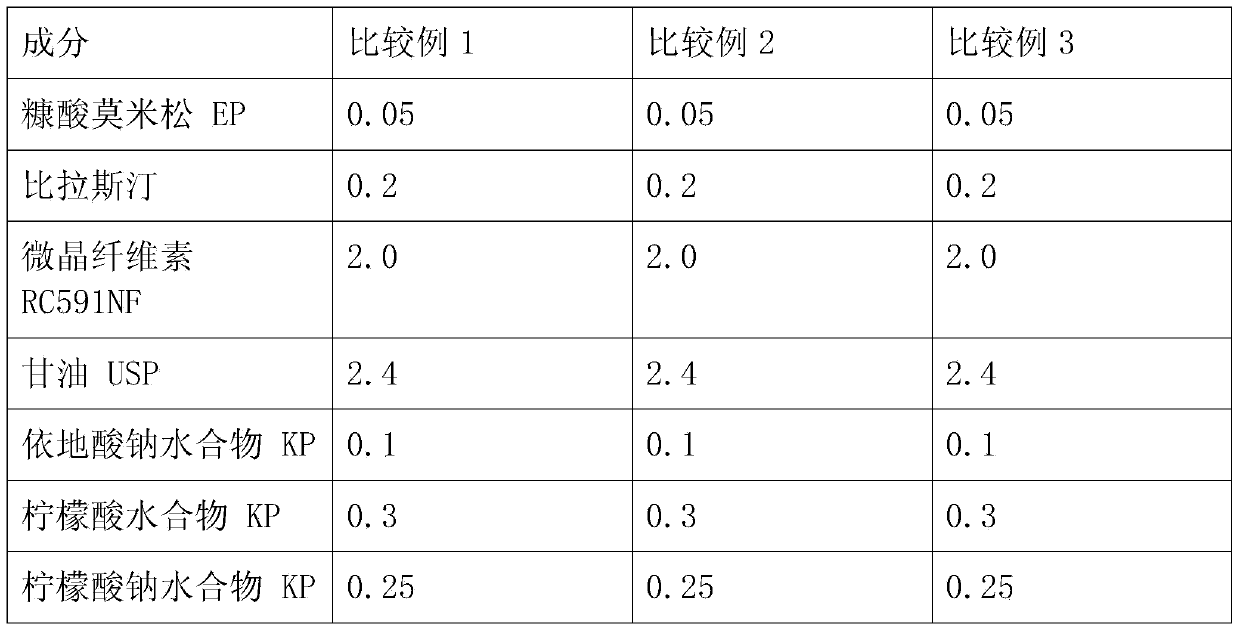
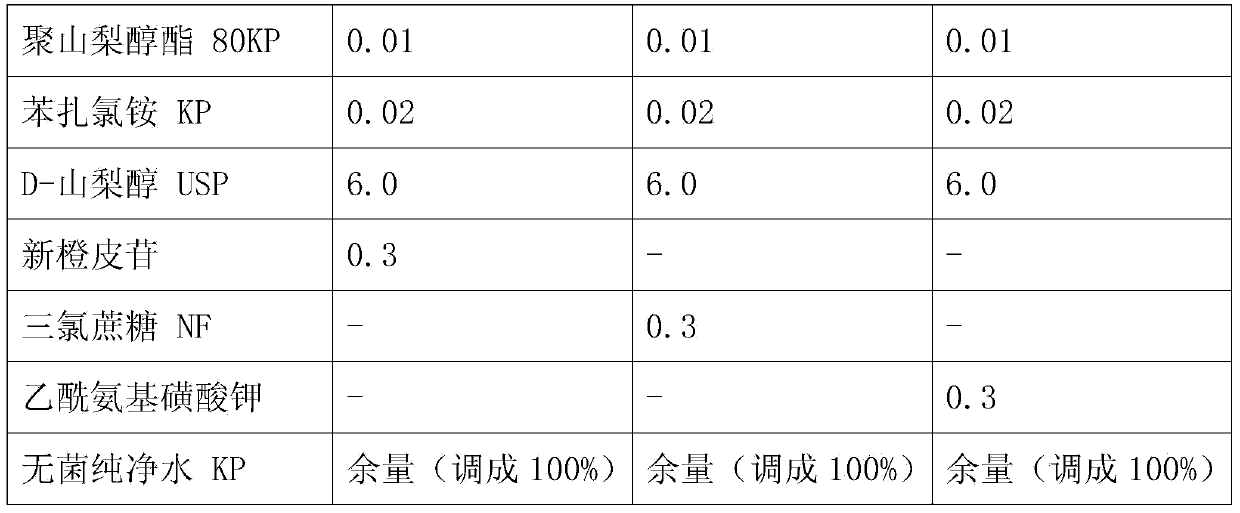
[0035] A nasal cavity spray suspension was prepared with the composition and content shown in Table 1 below. The contents in Table 1 represent w / v%.
[0036] Add microcrystalline cellulose RC591 and glycerin to sterile purified water, and disperse with a high-speed emulsifier (mixture 1). Add sterile purified water to other containers, and add bilastine, benzalkonium chloride, citric acid hydrate, sodium citrate hydrate, edetate sodium hydrate, D-sorbitol and thaumatin, Stirring was carried out to dissolve (mixture II). After adding sterilized purified water to another container, mometasone furoate and polysorbate 80 were added, followed by dispersion (Mixture III). After mixing and stirring the mixture I and the mixture II, it is mixed and stirred with the mixture III. Add sterile purified water to the resulting mixture to adjust the final volume.
[0037] Each specific embodiment component content of table 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com