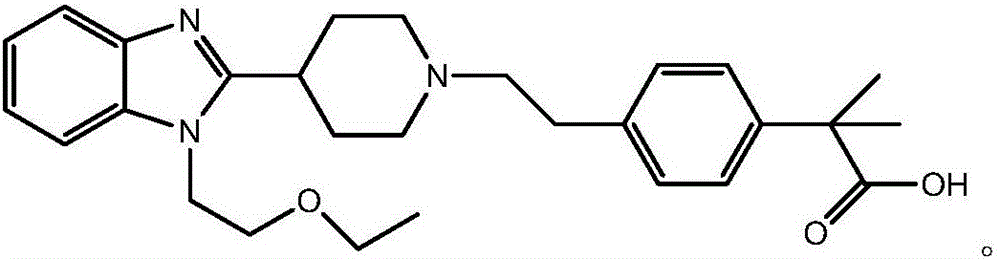
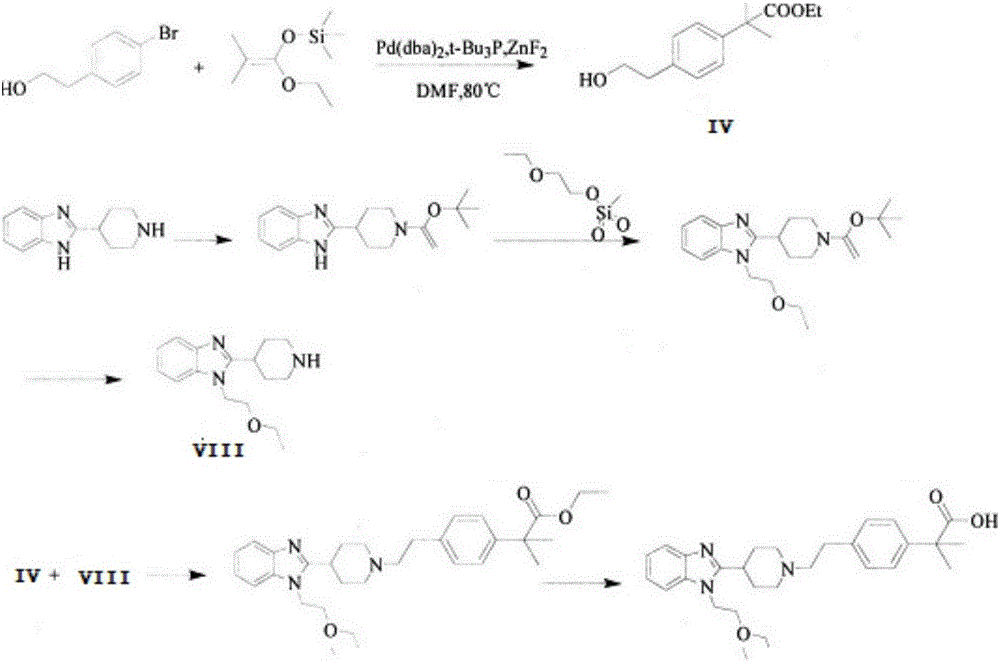
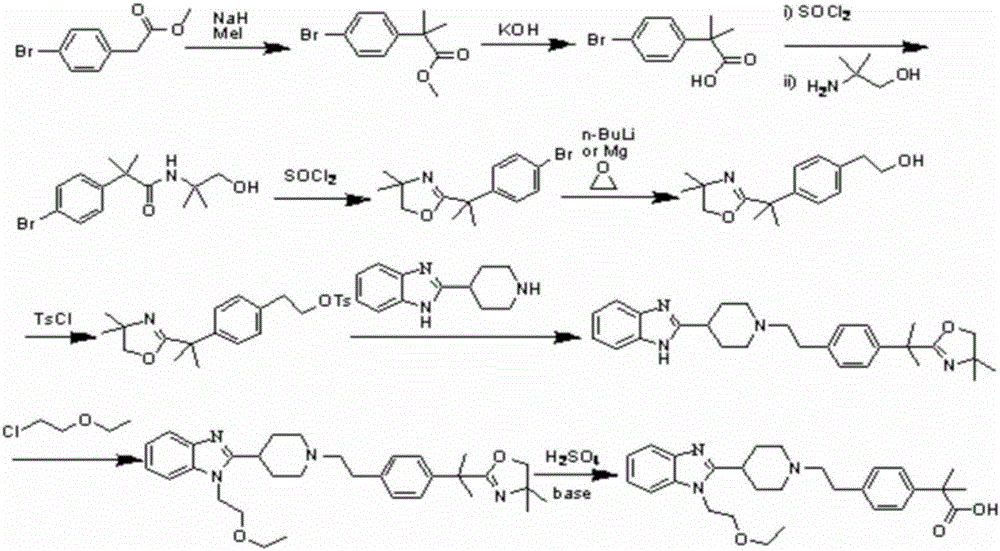
Bilastine preparation method
A technology of bilastine and molar ratio, which is applied in the field of preparation of bilastine, can solve the problems of cumbersome operation, harsh operating conditions, long route, etc., and achieve simple reaction operation, reduced reaction cost, high yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0049] Example 1-1: Synthesis of 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylic acid tert-butyl ester (compound 3)
[0050] Add 55.2g of 2-nitroaniline (compound 1, 0.4mol) and 85.3g of 1-Boc-piperidine-4-carbaldehyde (compound 2, 0.4mol) into a mixed solvent of ethanol (3.2L)-water (80ml), and dissolve completely Add 139.3gNa 2 S 2 0 4 (0.8mol), heated to reflux, TLC followed the reaction, the reaction was complete after 8 hours, the precipitate was filtered off, the filter cake was washed with absolute ethanol (1.6L×2), and the filtrates were combined and spin-dried to obtain a precipitate. After dissolving it in ethanol, it was recrystallized from ethanol-water to obtain, 101.3g tert-butyl 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylate (compound 3), yield 84 %.
Embodiment 1-2
[0051] Example 1-2: Synthesis of 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylic acid tert-butyl ester (compound 3)
[0052] Add 55.2g of 2-nitroaniline (compound 1, 0.4mol) and 102.4g of 1-Boc-piperidine-4-carbaldehyde (compound 2, 0.48mol) into a mixed solvent of ethanol (3.2L)-water (128ml), and dissolve completely Add 174.1gNa 2 S 2 0 4 (1mol), heated to reflux, TLC followed the reaction, the reaction was complete after 8 hours, the precipitate was filtered off, the filter cake was washed with absolute ethanol (1.6L×2), and the filtrates were combined and spin-dried to obtain a precipitate. After dissolving it in ethanol, it was recrystallized from ethanol-water to obtain, 108.5g tert-butyl 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylate (compound 3), yield 90 %.
Embodiment 1-3
[0053] Example 1-3: Synthesis of 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylic acid tert-butyl ester (compound 3)
[0054] Add 55.2g of 2-nitroaniline (compound 1, 0.4mol) and 110.9g of 1-Boc-piperidine-4-carbaldehyde (compound 2, 0.52mol) into a mixed solvent of ethanol (3.2L)-water (160ml), and dissolve completely Add 208.9gNa 2 S 2 0 4 (1.2mol), heated to reflux, TLC followed the reaction, the reaction was complete after 8 hours, the precipitate was filtered off, the filter cake was washed with absolute ethanol (1.6L×2), and the filtrates were combined and spin-dried to obtain a precipitate. After dissolving it in ethanol, it was recrystallized from ethanol-water to obtain, 103.7g tert-butyl 4-(1H-benzo[d]imidazol-2-yl)piperidine-1-carboxylate (compound 3), yield 86 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com