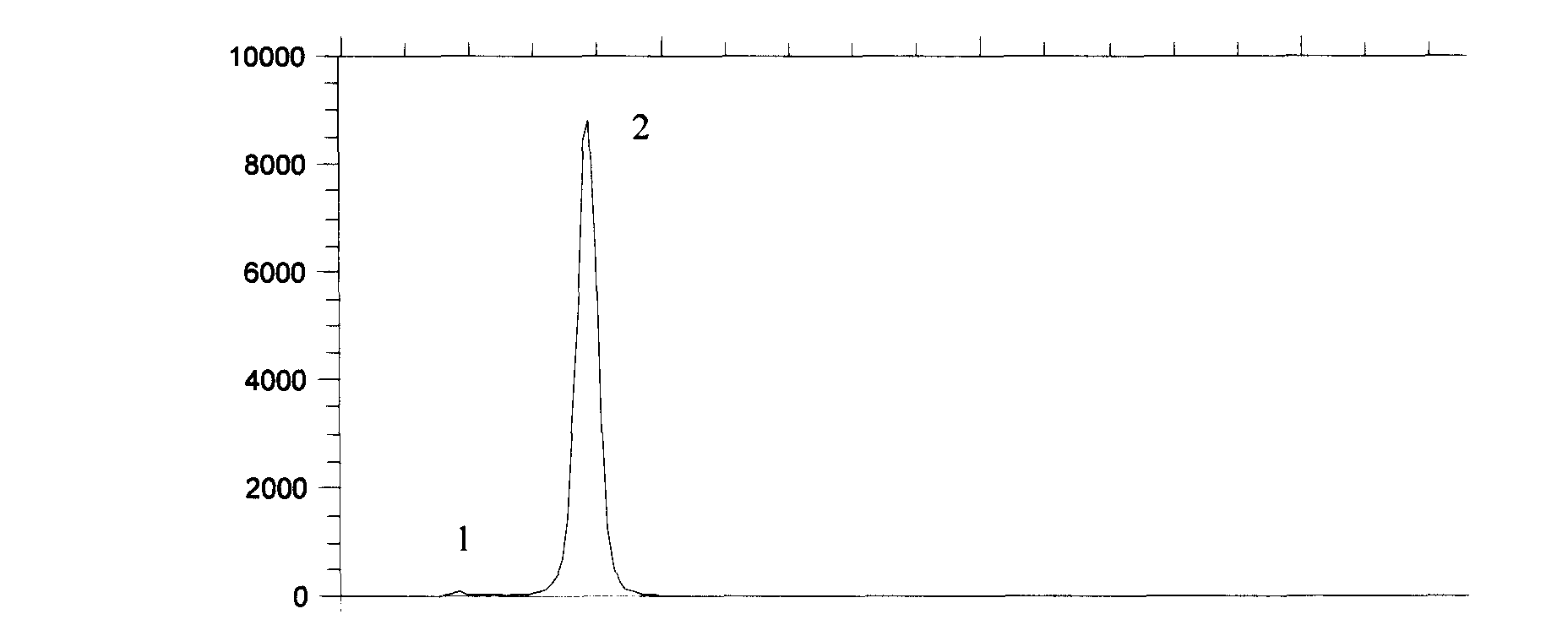
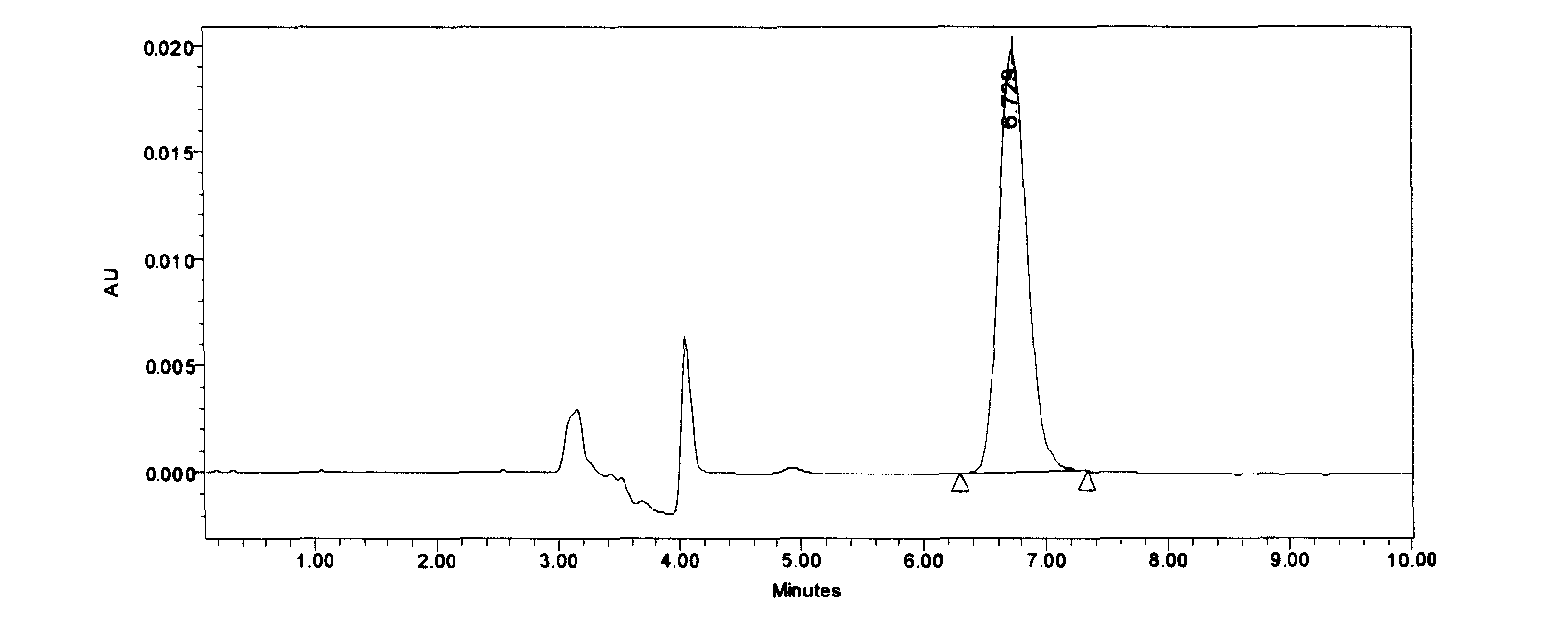
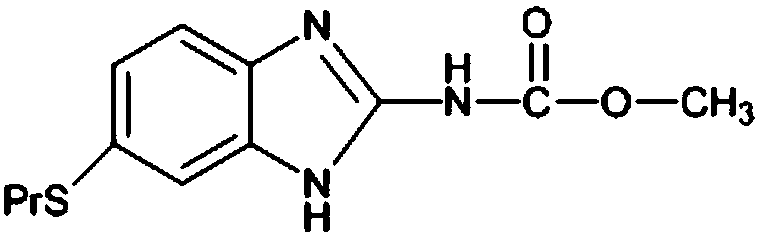
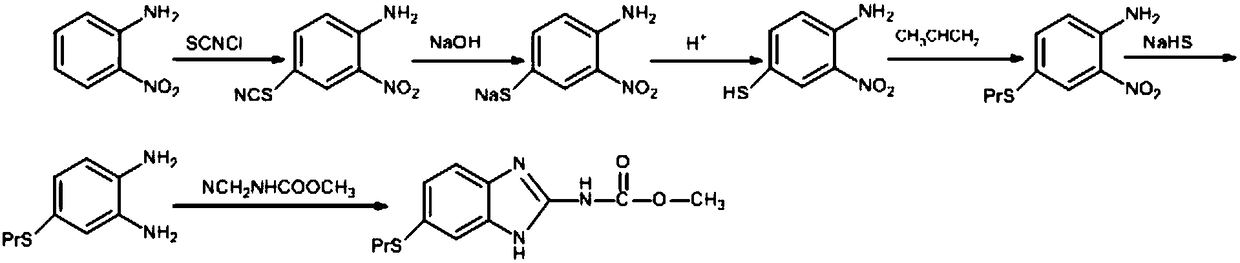
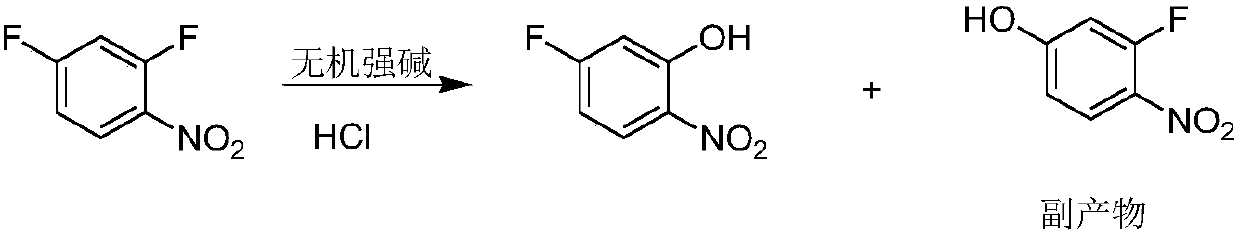
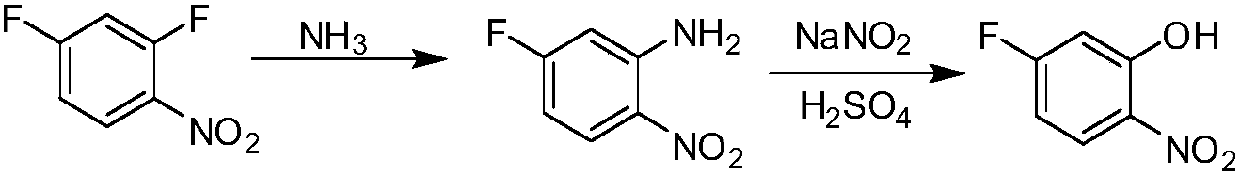
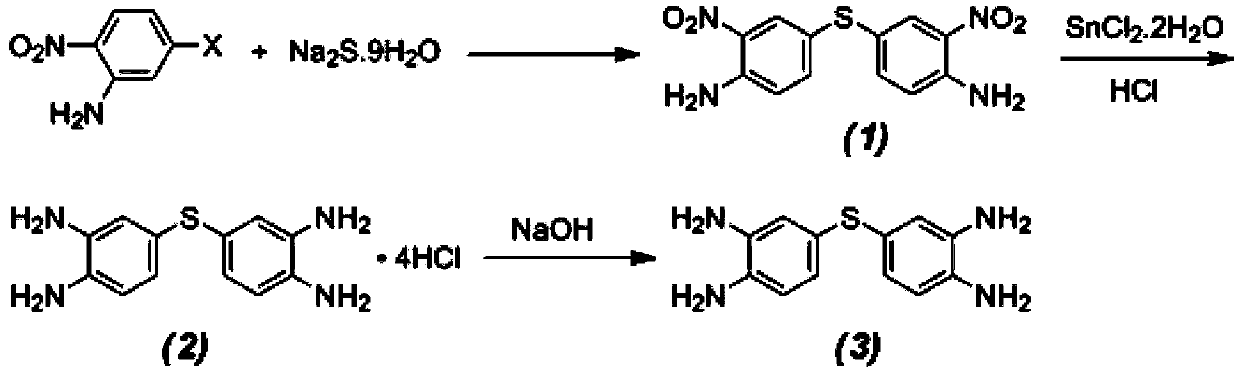
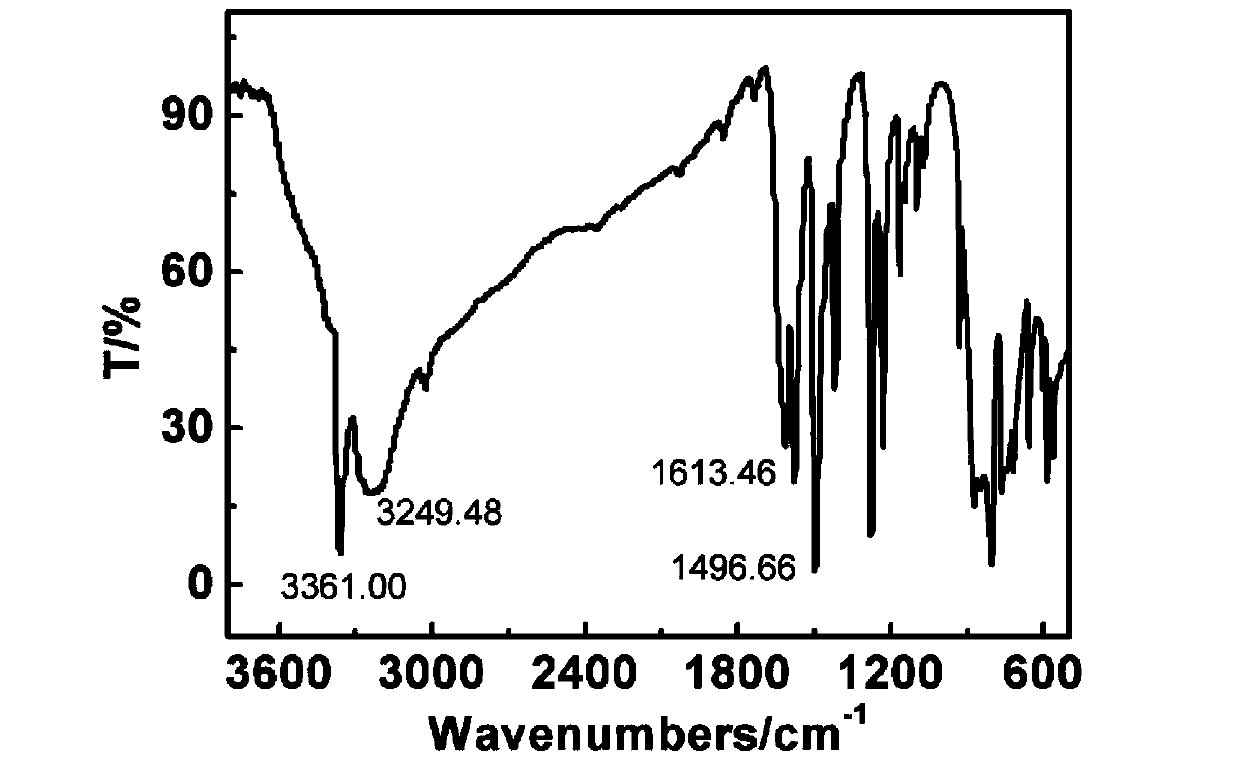
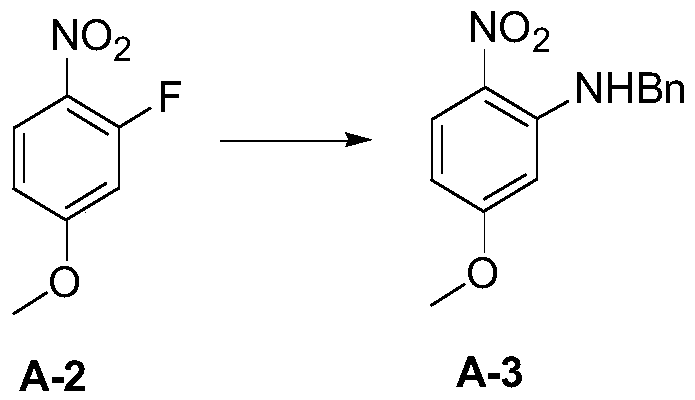
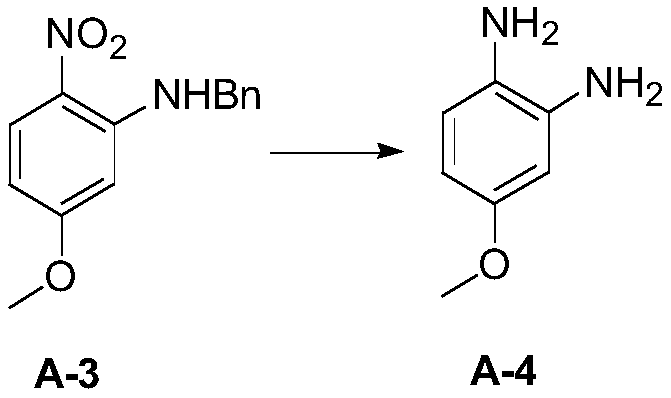
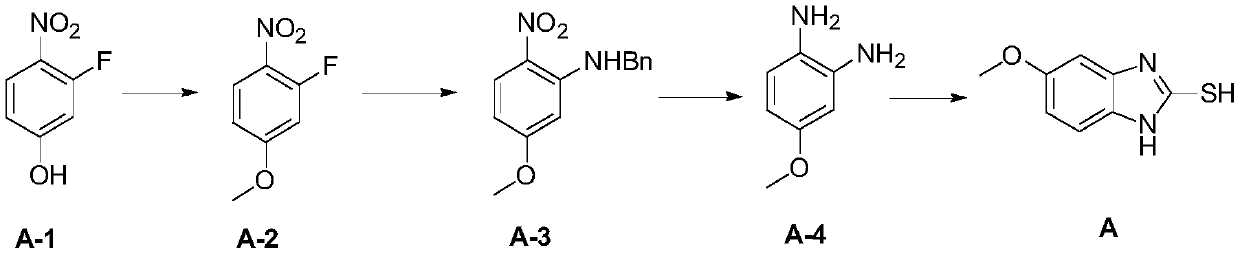
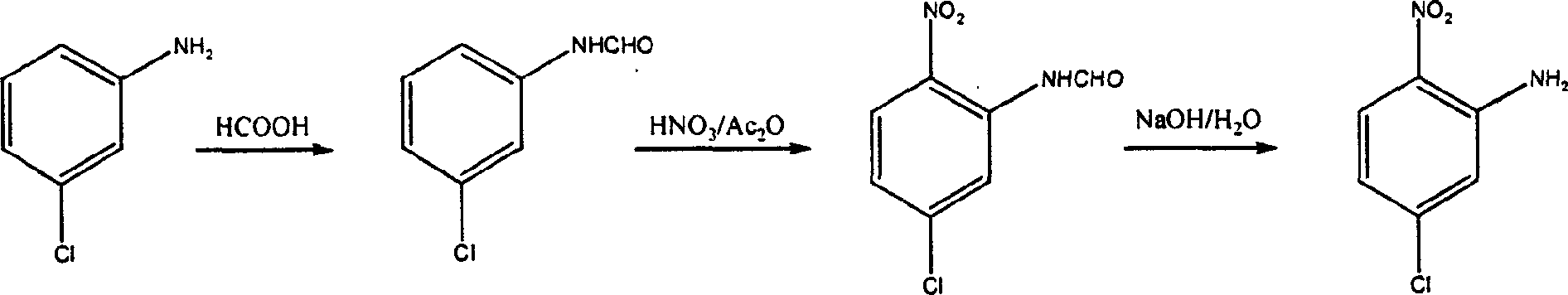
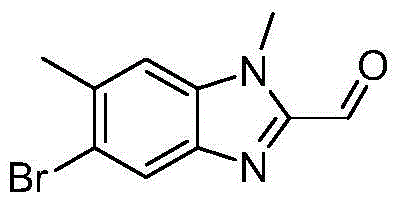
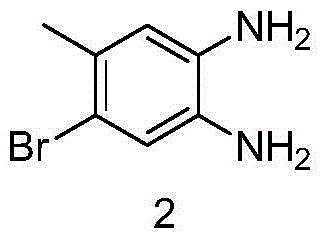
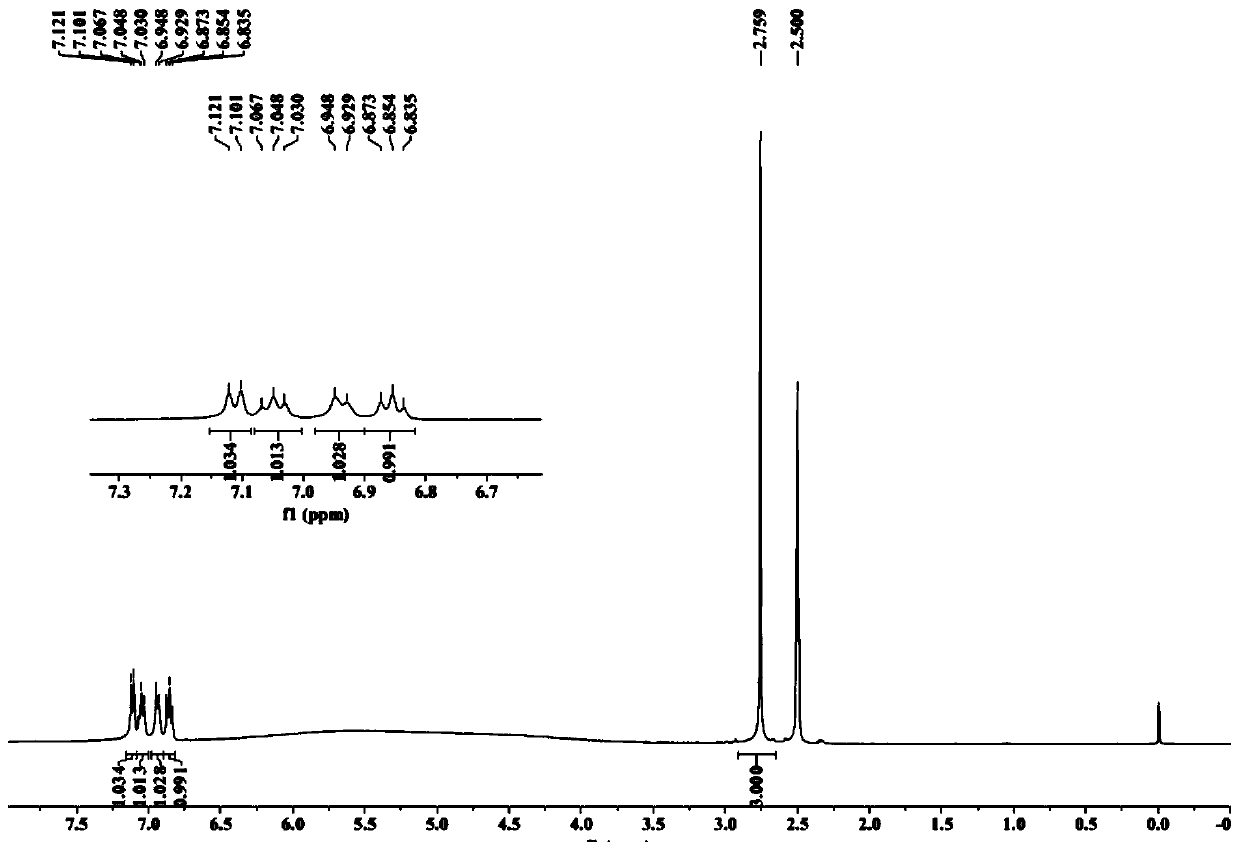
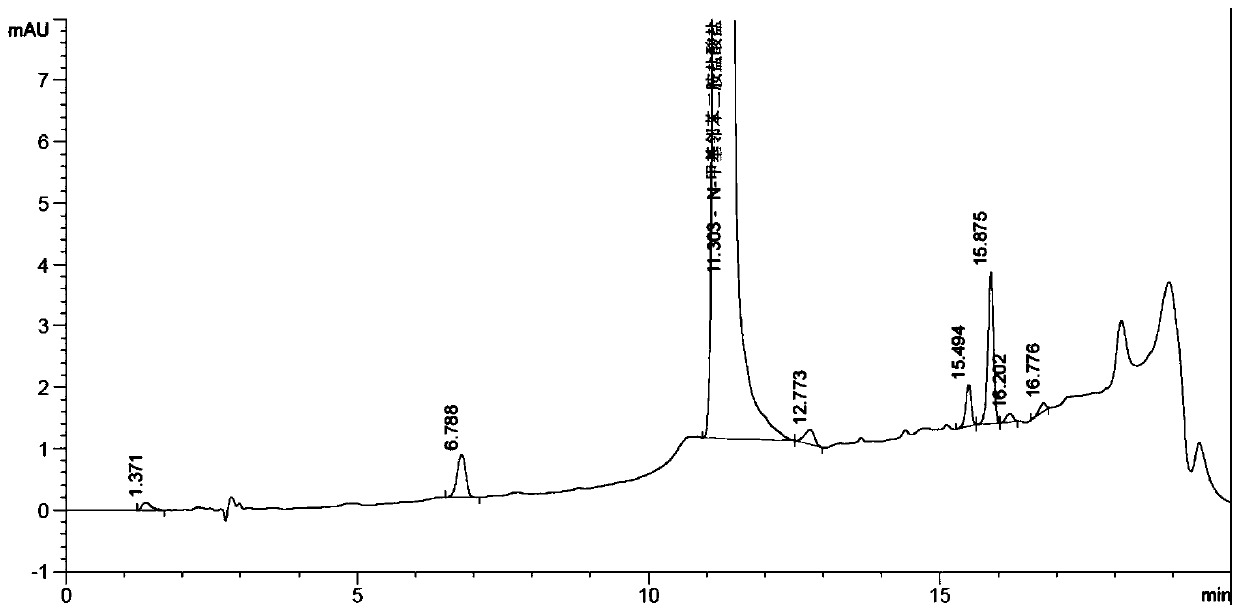
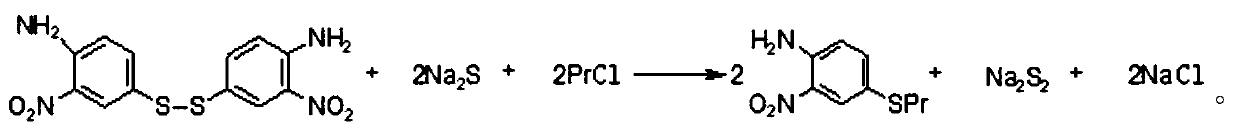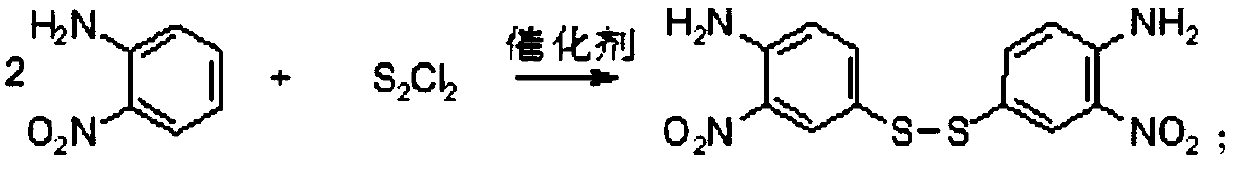Patents
Literature
53 results about "2-Nitroaniline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
2-Nitroaniline is an organic compound with the formula H₂NC₆H₄NO₂. It is a derivative of aniline, carrying a nitro functional group in position 2. It is mainly used as a precursor to o-phenylenediamine.
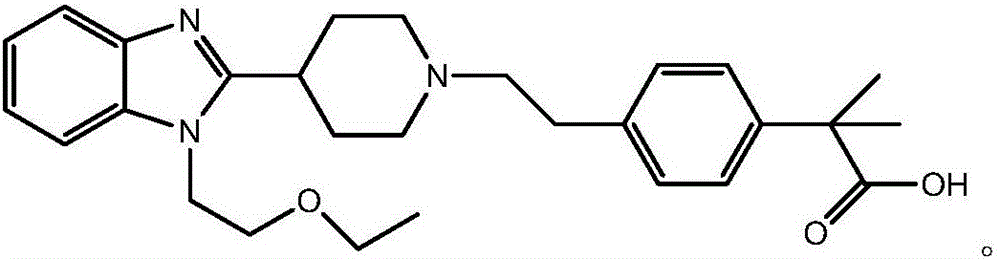
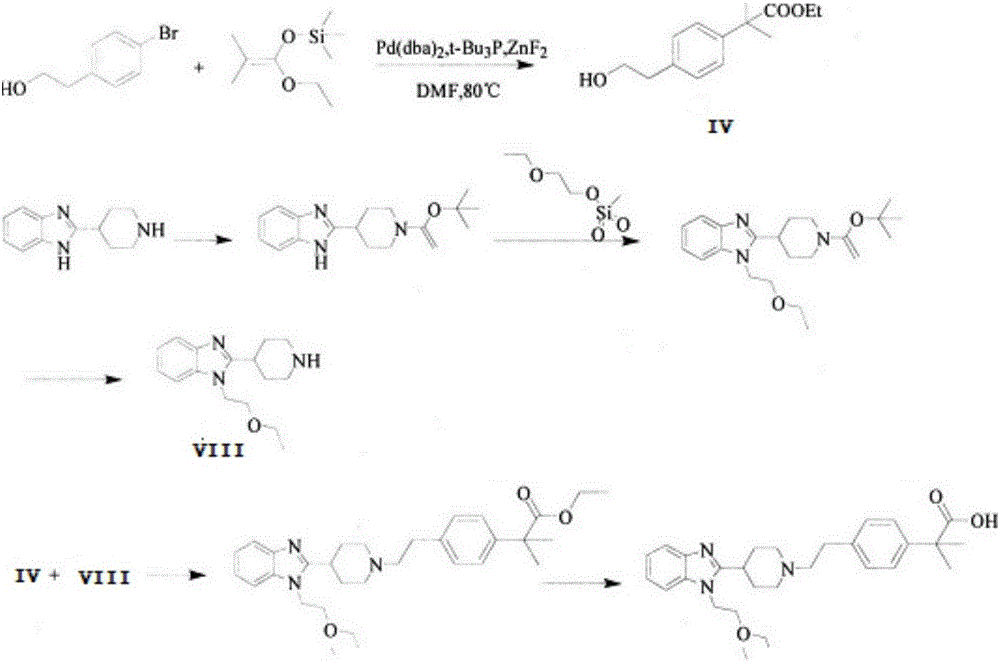
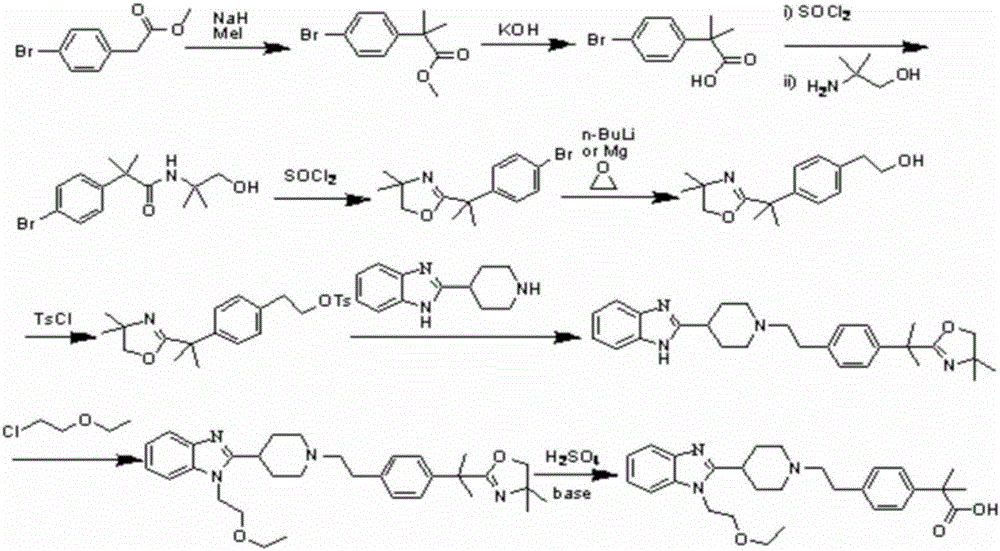
Bilastine preparation method
ActiveCN106146459ALower reaction costMild reaction conditionsOrganic compound preparationSulfonic acid esters preparationState of artAlkyl transfer
The invention discloses a Bilastine preparation method. The Bilastine preparation method includes that 2-nitroaniline which is low in price and easy to obtain is taken as a raw material which is subjected to reduction-n-cyclohexylmaleimide reaction, alkylation reaction, hydrolyzing and coupling prior to hydrolyzing to obtain Bilastine. With the method, shortcomings that harsh operation conditions, high toxicity, expensive raw materials and tedious operation in the prior art are overcome, reaction conditions in each step are moderate, and the synthetic method is simple in operation, easy to deal with, few in side products, high in yield and purity, low in production cost and suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Method for producing 5-chloro-2-nitroaniline
InactiveCN102531923ASimple methodEasy to operateOrganic compound preparationAmino compound preparationNitrationOil phase
The invention discloses a method for producing 5-chloro-2-nitroaniline. The method comprises the following steps of: performing nitration on m-dichlorobenzene by using mixed acid of sulfuric acid and nitric acid at the temperature of between 45 and 55 DEG C for 4 to 5 hours, then layering after the nitration is finished, washing an oil phase of the upper layer until the oil phase of the upper layer is neutral, performing soda boiling by using a 4 percent sodium hydroxide solution, standing and layering, washing an oil phase of a lower layer until the oil phase of the lower layer is neutral to obtain 2,4-dichloronitrobenzene; and adding 2,4-dichloronitrobenzene into a high-pressure amination kettle, introducing liquid ammonia, preserving heat at the temperature of between 140 and 150 DEG C under the pressure of 7.0 to 8.5MPa for 5 to 6 hours, relieving pressure until the pressure is 0.5MPa, pressing materials into a washing kettle, washing off ammonium chloride, and suction-filtering to obtain a filter cake containing a product 5-chloro-2-nitroaniline. The method is simple and convenient, and is easy to operate, and the content of a finished product reaches over 98 percent.
Owner:江苏优普生物化学科技股份有限公司
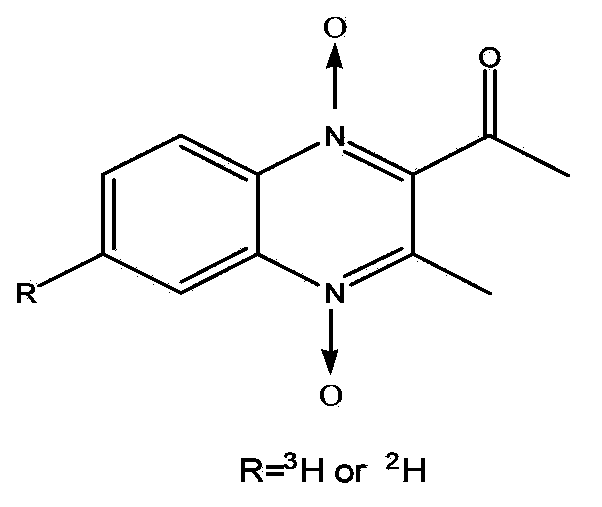
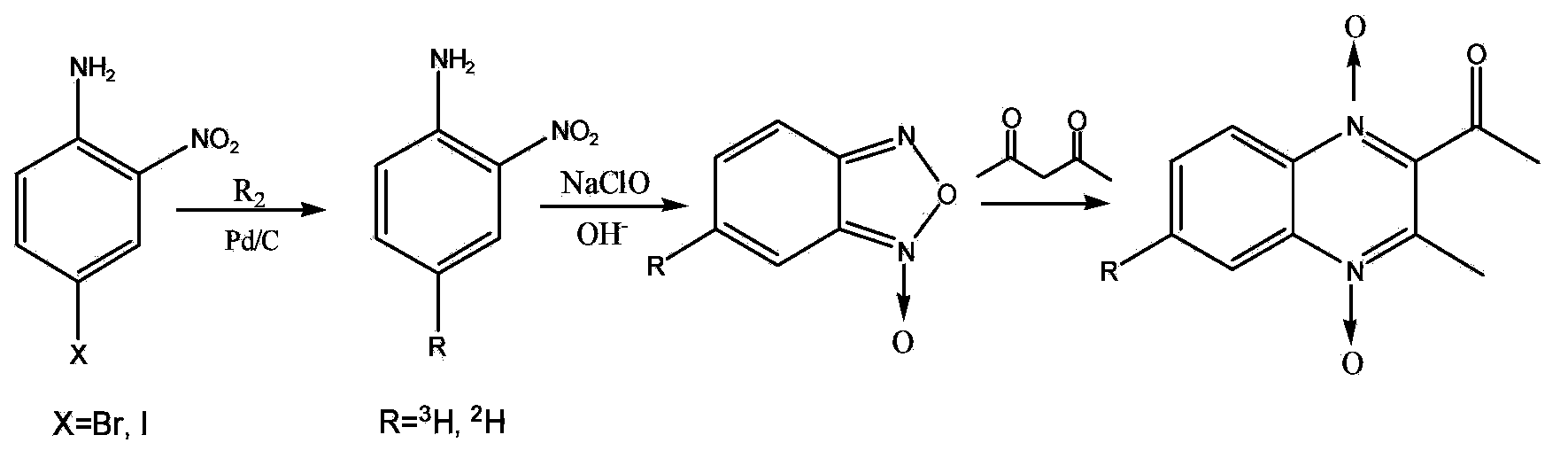
Preparation method of tritium or deuterium-labeled cyadox
InactiveCN101538249ARaw materials are easy to getReduce pollutionOrganic chemistryIn-vivo radioactive preparationsQuinoxalineDrug additive
The invention relates to a preparation method of a feed drug additive cyadox by tritium or deuterium labeling. The preparation method comprises the following steps: dehalogenating 4-bro-2-nitroaniline or 4-iodo-2-nitroaniline to exchange with tritium or deuterium in tritium gas or deuterium gas in the presence of a catalyst and an acid receptor to produce 4-[3]H-2-nitroaniline or 4-[2]H-2-nitroaniline; and preparing the tritium or deuterium-labeled cyadox by microsynthesis after an oxidation reaction, a Beirut reaction and a hydrazone forming reaction so as to obtain the tritium-labeled cyadox with high specific activity (12.63Ci / mmol), high radiochemical purity (above 98%) and high chemical purity (above 99.5%) or the deuterium-labeled cyadox with high chemical purity (above 99.5%). The method provides a material basis for systematic development of rules of absorption, distribution and metabolism of the cyadox in animals. The prepared 4-[3]H-2-nitroaniline or 4-[2]H-2-nitroaniline can be taken as a starting material for synthesizing all tritium or deuterium-labeled quinoxaline drugs without other substituents at the sixth place of a quinoxaline ring and is an important substance for synthesizing the tritium or deuterium-labeled drugs.
Owner:HUAZHONG AGRI UNIV
Albendazole synthesis method
InactiveCN109400537AAvoid it happening againNo pollution in the processOrganic chemistrySynthesis methodsSolvent
The invention discloses an albendazole synthesis method. The albendazole synthesis method includes reacting ammonium thiocyanate with chlorine gas in a lower alcohol solvent to obtain chlorothiocyanate, reacting ortho-nitroaniline with the chlorothiocyanate in the lower alcohol solvent to obtain 4-thiocyano-2-nitroaniline, reacting the 4-thiocyano-2-nitroaniline with a sodium hydroxide solution toobtain 4-sodium sulfonate-2-nitroaniline, performing hydrochloric acid acidification to obtain 4-mercapto-2-nitroaniline, subjecting the 4-mercapto-2-nitroaniline and propylene to Markovnikov addition reaction to obtain 4-propylthio-2-nitroaniline, and reacting 4-propylthio-o-phenylenediamine with methylcyanocarbamate to obtain albendazole. The albendazole synthesis method has the advantages thata novel synthetic route is applied to prepare the albendazole, the defects of high impurity quantity and low yield in the current production process are overcome, the chlorothiocyanate is prepared toserve as an intermediate and then reacts with the ortho-nitroaniline, and impurities can be avoided effectively; the propylene is introduced for the addition reaction, raw materials are clean and free from pollution, introduction of highly toxic sodium cyanide is avoided, the total yield is high, and the albendazole synthesis method has a good industrialization prospect.
Owner:SHANDONG GUOBANG PHARMA +1
Preparation method of fenbendazole
ActiveCN109467535AHigh available chlorineReduce usageOrganic chemistryChemical recyclingFenbendazoleNitration
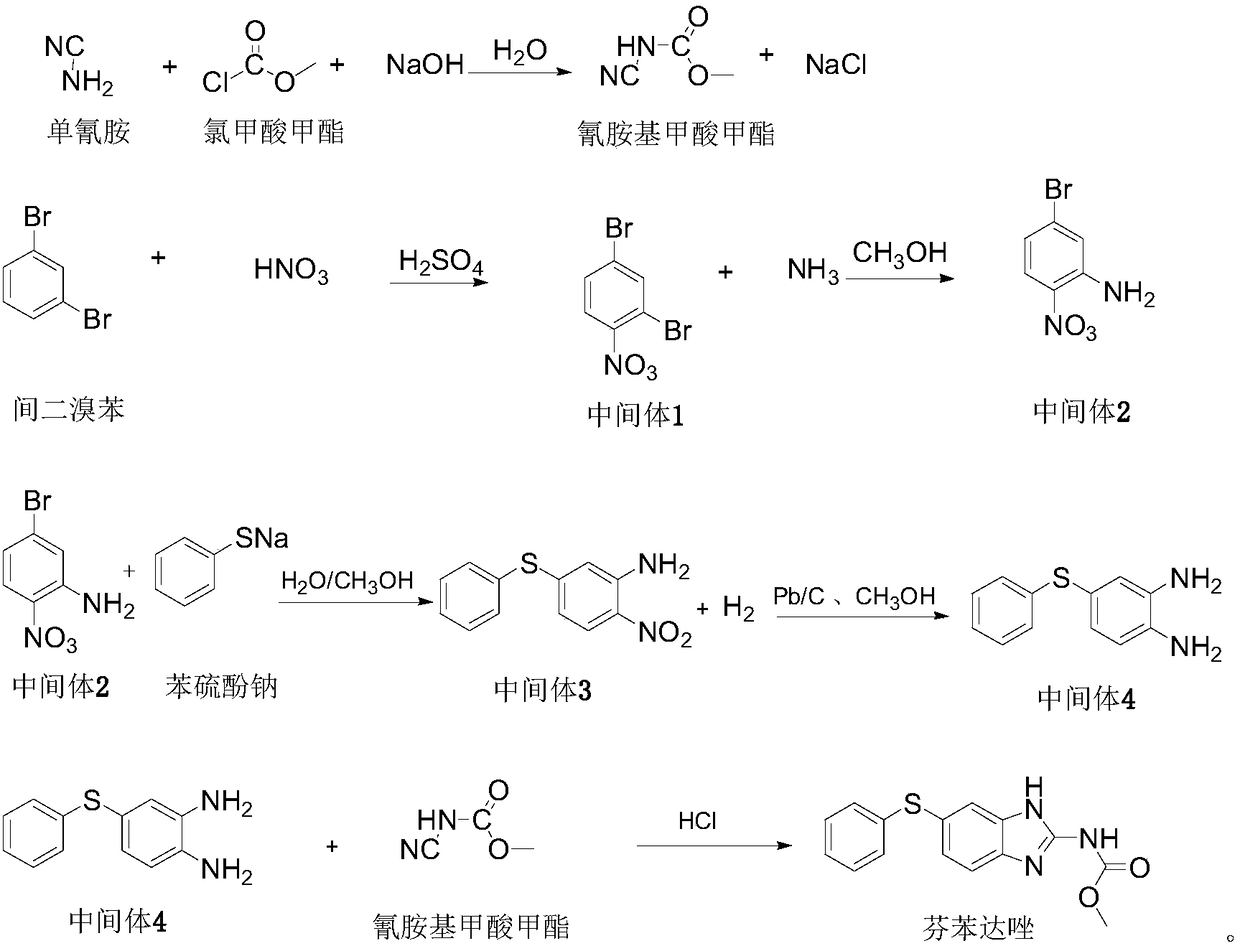
The invention discloses a preparation method of fenbendazole. The preparation method of fenbendazole is characterized by (1) taking m-dibromobenzene as a starting material, and nitrating a nitric acid / sulfuric acid system to prepare an intermediate 1(2,4-dibromonitrobenzene); (2) using the intermediate 1 as a raw material, and carrying out an ammoniation reaction with an ammonia methanol solutionto prepare an intermediate 2(5-bromo-2-nitroaniline); (3) taking the intermediate 2 and a sodium thiophenolate solution as raw materials, and carrying out condensation reaction to prepare an intermediate 3(4-phenylthio-2-nitroaniline); (4) carrying out hydrogenation reduction on the intermediate 3 through the catalysis of palladium charcoal to form intermediate 4(4-phenylthio-1,2-phenylenediamine); (5) carrying out cyclization reaction on the intermediate 4 and a methyl cyanamide aqueous solution to form the product, namely fenbendazole. The method is clean and environmentally friendly and lowin production cost, the purity of the product is more than 99.5%, and the yield is not less than 84.0%.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Triazinyl nitroxide and its synthesis and use
InactiveCN1887878AReasonable designSimple and fast operationOrganic chemistryAntineoplastic agentsSide chain2-Nitroaniline
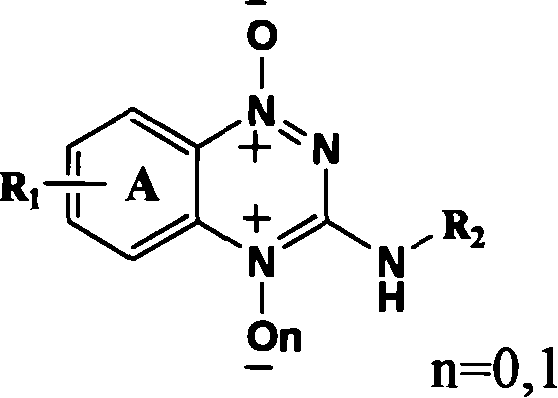
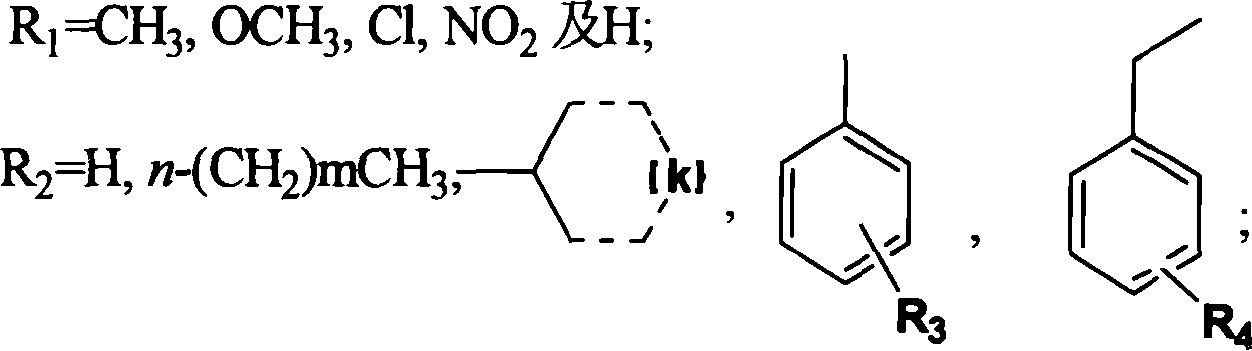
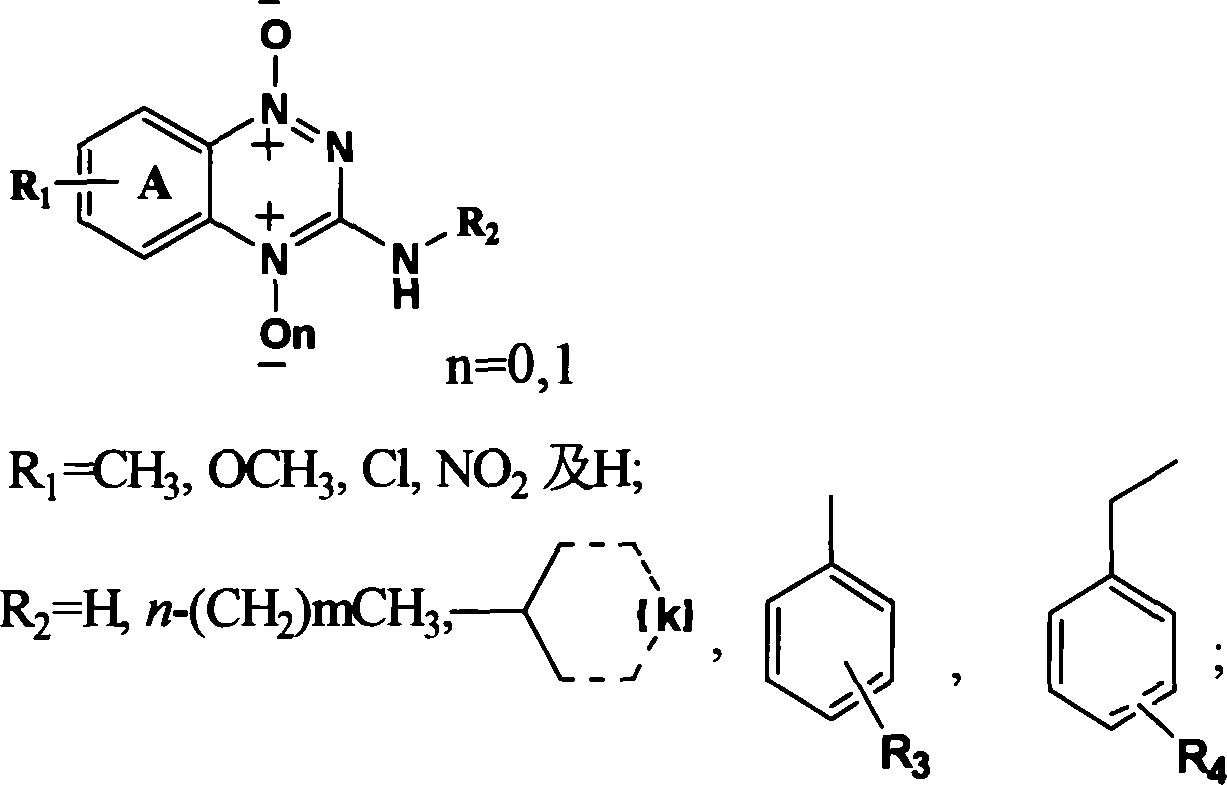
The present invention provides 3-substituted amino-1, 2, 4-substituted phentriazine-1, 4-dioxides in the general expression as shown. The compounds are prepared with substituted one-nitroaniline as main material and through acylation to produce urea, cyclization, chlorination, amino substitution, oxidation and other steps. The tests show that the compounds of the present invention have anticancer activity and hypoxia selectivity higher than those of TPZ and may be used in preparing hypoxia selective anticancer medicine.
Owner:ZHEJIANG UNIV
Chemical synthesizing method for 5-chlorine-2-nitroaniline
InactiveCN1418861AAtom utilization is highImprove protectionOrganic compound preparationAmino compound preparationChemical synthesis2-Nitroaniline
The method for synthesizing 5-chloro-2-nitrophenylamine uses 3-chlorophenylamine as initial raw material, and utilizes three-step reaction of formylation, nitration and hydrolysis to synthesize object compound 5-chloro-2-nitrophenylamine, its total yield rate is above 60% and its product purity is above 98%.
Owner:ZHEJIANG UNIV OF TECH
Process for producing 2-methyl-8-nitryl quinoline
The invention provides a method for preparing 2-methyl-8-nitroquinoline. The method comprises the following steps: o-nitroaniline reacts in hydrochloric acid and benzene solution to form hydrochloride of the o-nitroaniline; the o-nitroaniline hydrochloride and aldehyde are subjected to cyclization at a temperature between 10 and 100 DEG C; oxidation reaction is performed by use of oxidant at a temperature between 10 and 100 DEG C; and finally the 2-methyl-8-nitroquinoline with high purity is obtained through separation, neutralization, washing and drying. The method has the advantages that the method uses low-toxicity paraldehyde as a raw material to replace virulent crotonaldehyde, and uses iodine as the oxidant to replace virulent arsenic acid so as to reduce toxic effects on human and environment; inorganic acid and inorganic base used in the reaction are common acid and base; and the method is low in cost, little in pollution and high in product purity, and reaches the yield of 80 percent.
Owner:CINIC CHEM SHANGHAI
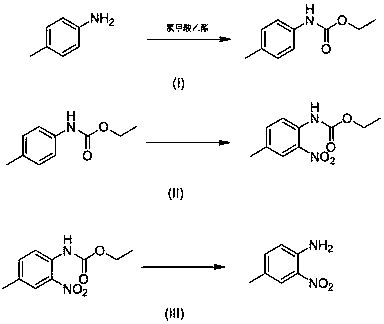
4-methyl-2-nitroaniline synthesis method
InactiveCN107759479AMild reaction conditionsNo need for high temperatureCarbamic acid derivatives preparationOrganic compound preparationNitrosoMethylaniline
The invention discloses a 4-methyl-2-nitroaniline synthesis method, which comprises: carrying out amino protection with ethyl chloroformate by using 4-methylaniline as a raw material to generate N-(p-toluene)ethyl carbamate; adding an oxidant and a copper salt catalyst, and carrying out a reaction for a certain time at a temperature of 50-120 DEG C in a reaction solvent by using a nitroso-containing compound as a nitrating agent to prepare a corresponding protected o-nitro-p-toluidine; and carrying out hydrolysis to obtain the target product. According to the present invention, the method hasadvantages of simple preparation process, mild reaction condition, high yield and environment protection.
Owner:NANJING UNIV OF SCI & TECH
Preparation method for tritium or deuterium labeled mequindox
ActiveCN103420929AHigh yieldSynthetic conditions are suitableOrganic chemistrySynthesis methodsVeterinary Drugs
The invention belongs to the technical field of veterinary drug preparation, and particularly relates to a preparation method for a tritium and deuterium labeled veterinary drug mequindox. The method comprises the following steps: adopting a trace synthesis method to allow tritium gas or deuterium gas and 4-bromine-2-nitroaniline or 4-iodine-2-nitroaniline to be subjected to dehalogenation reaction under the action of a catalyst and an acid acceptor, and meanwhile, introducing tritium gas or deuterium gas alternatively to generate 4-3H-2-nitroaniline or 4-2H-2-nitroaniline; performing the oxidation reaction and the Beirut reaction to prepare the C-6 tritium labeled or deuterium labeled mequindox, wherein the obtained product is clear in labeling point, high in specific activity (12.63 Ci / mmol), high in radiochemical purity (more than 98 percent) and high in chemical purity (more than 99.5 percent). The prepared tritium labeled mequindox provides a material foundation for the research of absorption, distribution, metabolism and residue elimination rules of the mequindox in an animal body; the prepared deuterium labeled mequindox can serve as an internal standard substance for the quantitative analysis of a mequindox trace amount.
Owner:HUAZHONG AGRI UNIV
Quinoxaline-N1,N4-dioxide derivative capable of inhibiting activity of DNA topoisomerase, preparation method and application of quinoxaline-N1,N4-dioxide derivative
PendingCN110551072AHas inhibitory activityStrong inhibitory activityAntibacterial agentsOrganic chemistryQuinoxalineStaphylococcus aureus
The invention belongs to the technical field of biochemistry, and particularly relates to a quinoxaline-N1,N4-dioxide derivative capable of inhibiting the activity of DNA topoisomerase, a preparationmethod and application of the quinoxaline-N1,N4-dioxide derivative. 4,5,-difluoro-2-nitroaniline is used as a raw material for synthesis of the quinoxaline-N1,N4-dioxide derivative, the quinoxaline-N1,N4-dioxide derivative reacts with sodium hypochlorite under catalysis of a basic catalyst, namely sodium hydroxide, and 5,6-difluoro-N-benzofuroxan is obtained; and then the quinoxaline-N1,N4-dioxidederivative reacts with different substrates in Beirut reaction and substitution reaction, and a series of the quinoxaline-N1,N4-dioxide derivative is obtained. According to the quinoxaline-N1,N4-dioxide derivative, the preparation method and application of the quinoxaline-N1,N4-dioxide derivative, quinoxoline-N1,N4-dioxide has good bacteriostatic activity to previously reported gram-negative bacteria, and also had good bacteriostatic activity to actinobacilluspleuropneumoniae and gram-positive bacteria such as staphylococcus aureus and streptococcus pneumoniae.
Owner:HUAZHONG AGRI UNIV
Synthesis method and purification method of 5-iodo-2-methylbenzimidazole
InactiveCN102936223AGood atom economySimple and fast operationOrganic chemistryPurification methodsOrganic synthesis
The invention discloses a synthesis method and purification method of 5-iodo-2-methylbenzimidazole, relating to the field of organic synthesis. The method comprises the following steps: by using o-nitroaniline as a main raw material, simple substance iodine as an iodine source and oxydol as an oxidizer, carrying out iodination reaction in a polar solvent in the presence of sulfuric acid to obtain 4-iodo-2-nitroaniline; and in the polar solvent, carrying out hydrogenation reduction by using Raney nickel as a catalyst to obtain 4-iodo-1,2-phenylenediamine, and carrying out cyclization reaction by using slight acetic acid as a catalyst and ortho-triacetate as a cyclization reagent to obtain 5-iodo-2-methylbenzimidazole, and finally, carrying out decolorization and purification in an acetate generation mode. The synthesis method disclosed by the invention belongs to green synthesis, has the advantages of high atomic economical efficiency and less waste, and is simple to operate; the catalyst has the advantages of low price, small recovery loss and less waste, and is simple to operate; and the product decolorization and purification mode is simple and efficient, the product appears a creamy white powder solid, and the purity is higher than 99.5%.
Owner:JIANGSU ZHONGDAN PHARMA RES +1
Preparation method for 5-chloro-2-nitroaniline
InactiveCN108329211AEasy to cleanReduce pollutionAmino preparation by hydrogen substitutionNitro compound preparationFiltrationNitration
The invention discloses a preparation method for 5-chloro-2-nitroaniline. The preparation method comprises the following steps: performing nitration by using m-dichlorobenzene as a starting material to prepare 2,4-dichloronitrobenzene, adding the 2,4-dichlorobenzene and a solvent into an autoclave, adding ammonia, performing high-pressure amination, after the amination is completed, performing cooling, performing pressure filtration, removing the solvent from a filtrate, and performing refining to obtain the target product 5-chloro-2-nitroaniline. The method provided by the invention has the following advantages: (1) nitrogen dioxide is used as a nitrating reagent to replace a traditional nitric acid-sulfuric acid mixed acid nitrating agent, so that no waste acid is produced, cleanliness of an industrial synthesis reaction is improved, and environmental pollution is reduced; and (2) after the amination is completed, by-product ammonium chloride is directly removed by pressure filtration, so that an amount of waste water is small, and an amination yield is high.
Owner:江苏优普生物化学科技股份有限公司
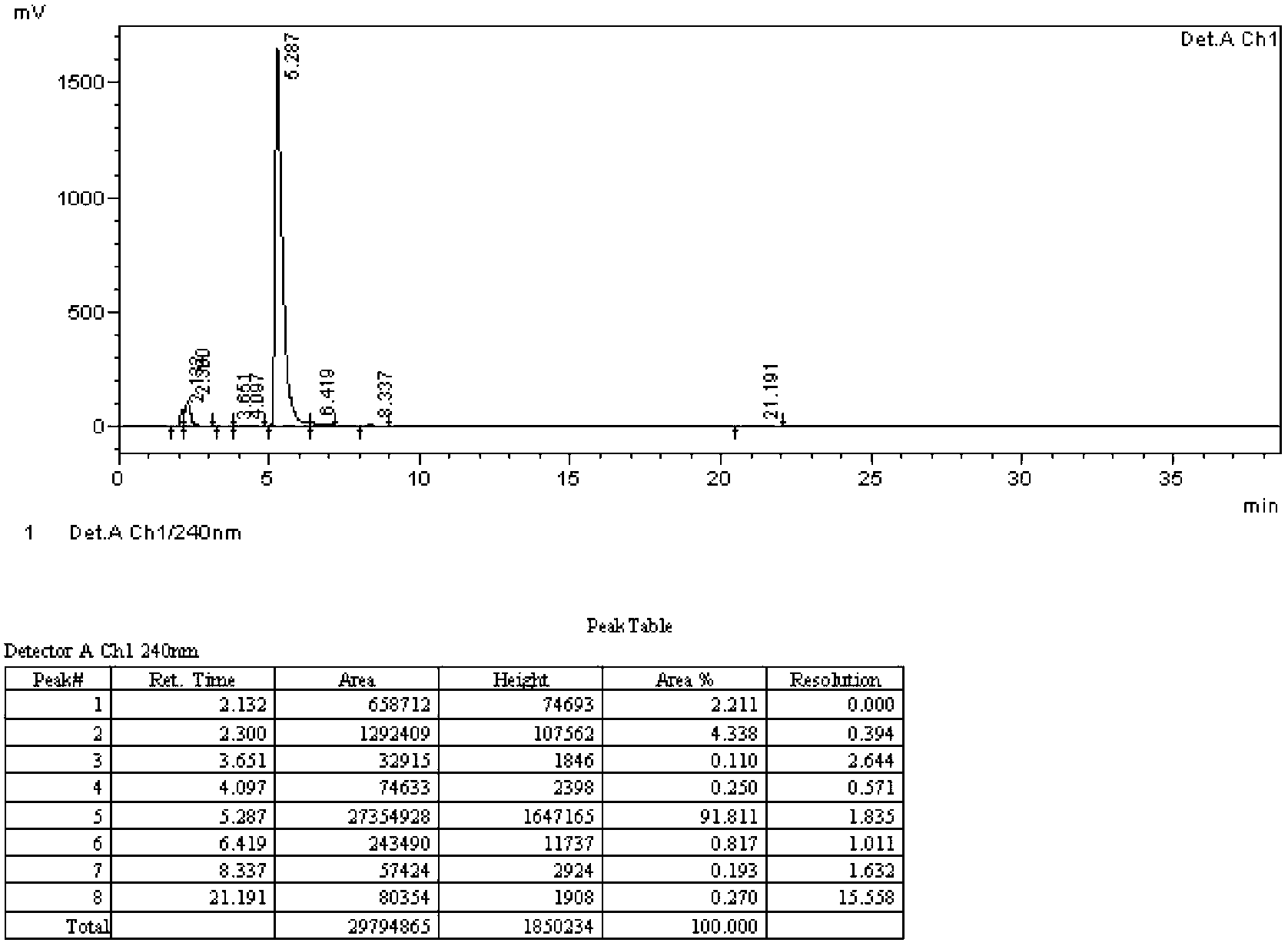
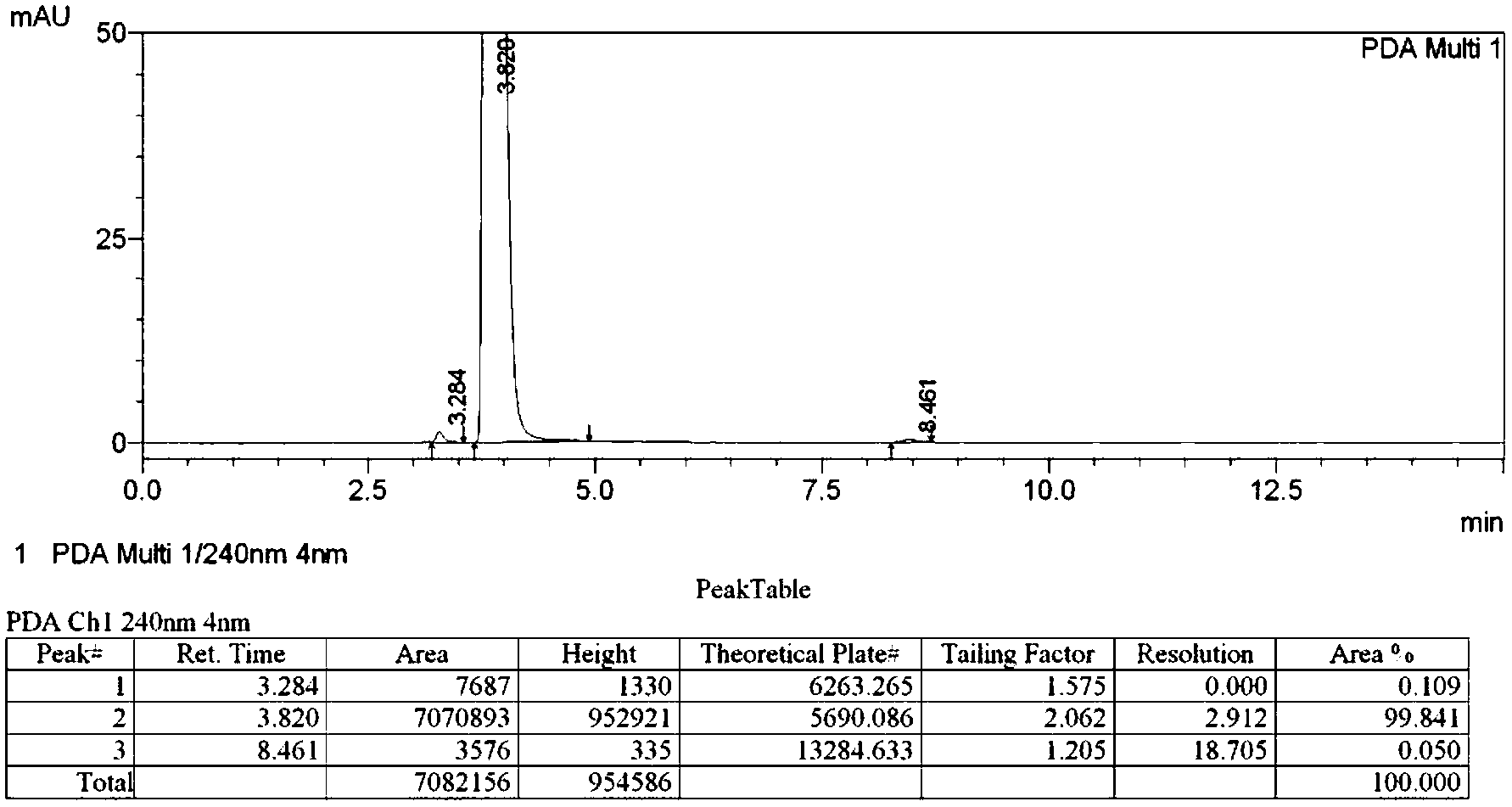
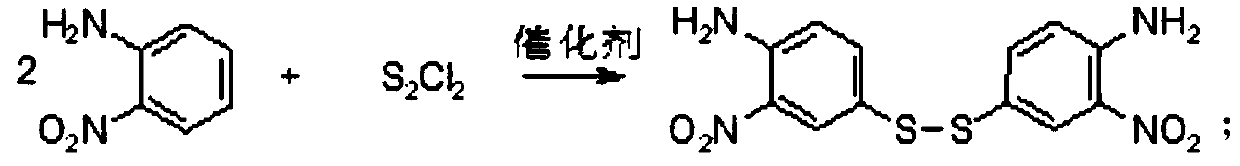
Preparation method of 4-propythio-2-nitroaniline
ActiveCN110498752AHigh yieldImprove securityHydropoly/poly sulfide preparationSulfide preparationAlkyl transfer2-Nitroaniline
The invention discloses a preparation method of 4-propythio-2-nitroaniline, and solves the technical problems of unreasonable preparation method, high raw material price, high toxicity, complex operation, high cost, large production of three wastes, low yield and unsuitability for industrial production in the prior art. The preparation method of 4-propythio-2-nitroaniline provided by the inventiontakes o-nitroaniline, disulfur dichloride and chloropropane as the raw materials, and carries out condensation reaction and alkylation reaction in order under the action of a catalyst so as to obtain4-propythio-2-nitroaniline. The method can be widely applied technical field of synthesis of albendazole.
Owner:SHANDONG GUOBANG PHARMA +1
Reusable transition metal complex catalyst useful for the preparation of high pure quality 3,3′-diaminobenzidine and its analogues and a process thereof
ActiveUS7999112B2High yieldRhodium organic compoundsMercury organic compounds2-NitroanilinePhotochemistry
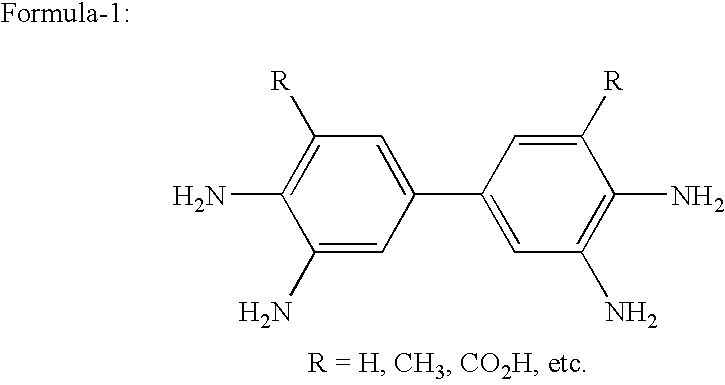
The present invention provides a reusable transition metal complex catalyst useful for the preparation of high pure quality 3,3′-diaminobenzidine and its analogues. The present invention also provides to a process for the preparation of a reusable transition metal complex catalyst. The present invention further provides a process for the preparation of 3,3′-diaminobenzidine (DAB) or 3,3′,4,4′ Tetraminobiphenyl (TAB) using reusable transition metal complex catalyst. The high quality 3,3′-diaminobenzedine (DAB) and its analogues are prepared by coupling 4-halo-2-nitroaniline to 3,3′-dinitrobenzidine (DNB) using transition metals as catalysts followed by reduction of 3,3′-dinitrobenzidine to the corresponding substituted 3,3′-diaminobenzidine of formula 1 in high yields.
Owner:COUNCIL OF SCI & IND RES
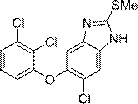
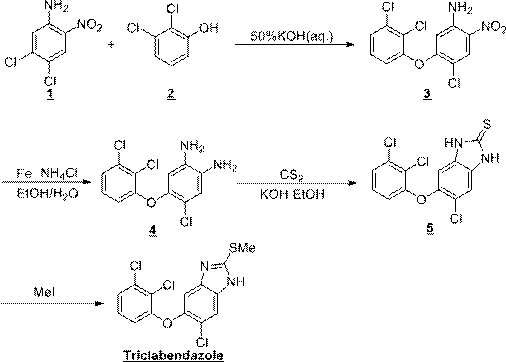
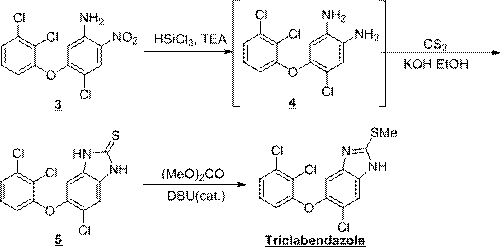
Preparation method of triclabendazole
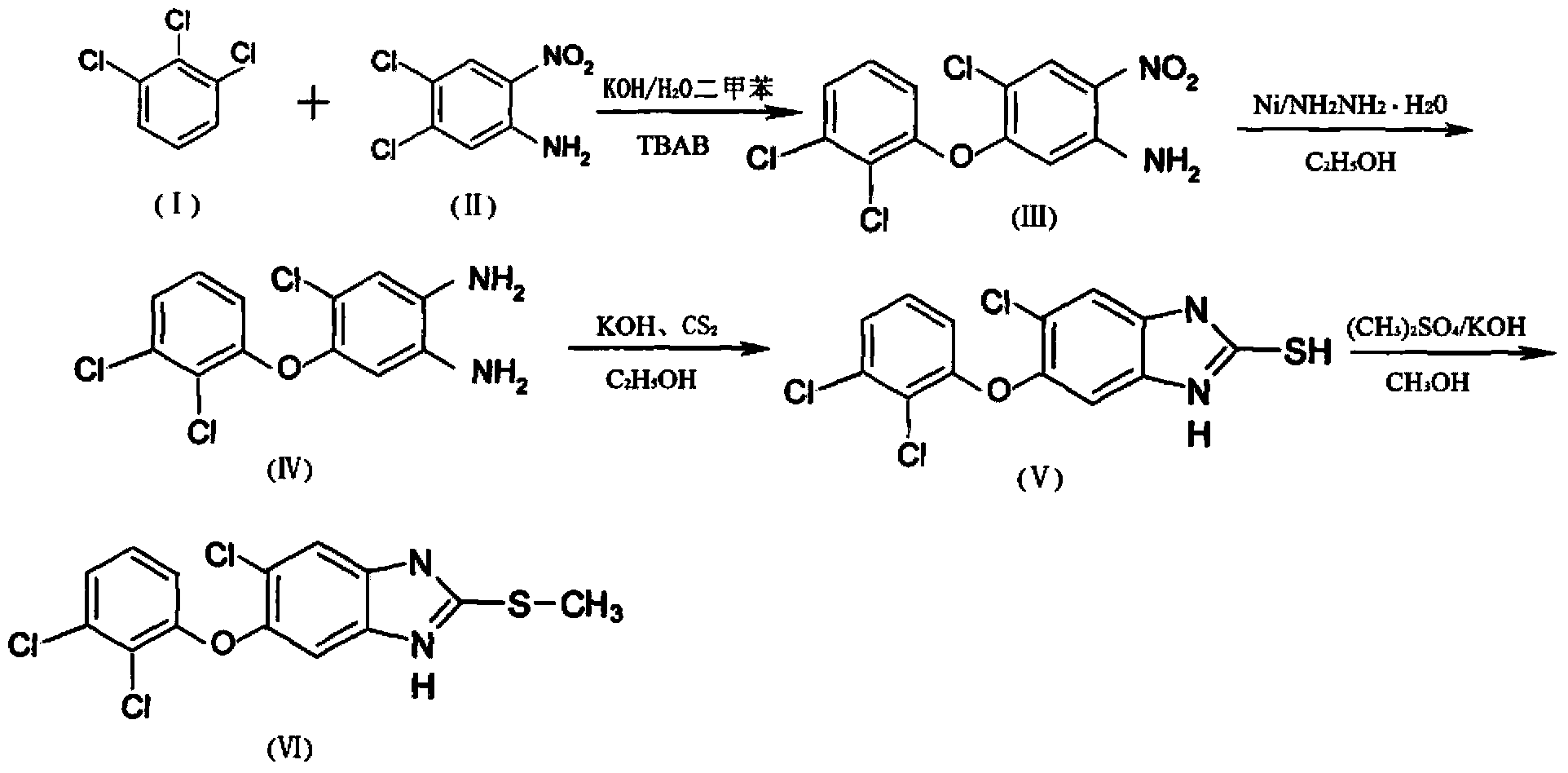
The invention discloses a method for synthesizing triclabendazole by taking 1,2,3-trichlorobenzene as a raw material. The triclabendazole is generated through three steps by taking the 1,2,3-trichlorobenzene as a starting raw material. The method has the advantages that the inexpensive 1,2,3-trichlorobenzene is used as the starting raw material, and expensive 2,3-dichlorophenol with strong sensitization is not used. The 1,2,3-trichlorobenzene is hydrolyzed in high-concentration alkali liquor to prepare 2,3-dichlorophenol sodium which reacts with 4,5-dichloro-2-nitroaniline in a methylbenzene aqueous solution, and free 2,3-dichlorophenol is not generated again, so that the reaction yield is improved, and pollutions generated during production are avoided. The 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline is reduced by adopting a hydrogen catalytic transfer method, so that a large amount of iron mud which is difficult to treat and pollutes the environment is not generated; and an adopted hydrogen donator is low in price and does not cause any pollution to the environment, so that the triclabendazole is suitable for large-scale industrial production.
Owner:CHANGZHOU JIALING MEDICINE IND
Method for hydrogenation reduction of 4-thiophenyl-2-nitroaniline through raney nickel
The invention discloses a method for hydrogenation reduction of 4-thiophenyl-2-nitroaniline through raney nickel. The method is characterized in that reducing 4-thiophenyl-2-nitroaniline into 4-thiophenyl o-phenylenediamine is one of the necessary operations in the technology of producing fenbendazole at present. According to the invention, the method adopting hydrogenation reduction is provided to replace the existing reduction method, so the cost is reduced, and the energy saving and environmental protection can be ensured.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
5-fluoro-2-nitrophenol preparation method
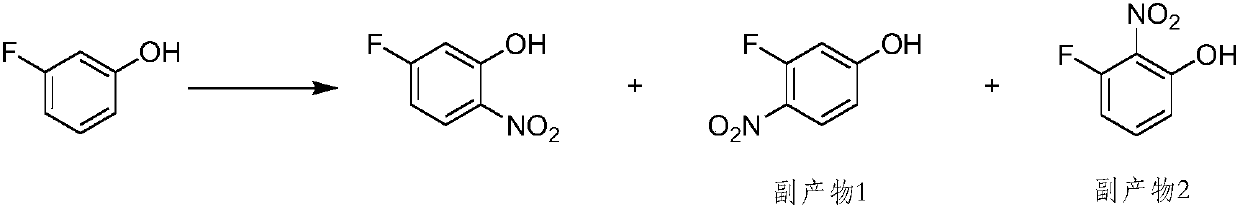
ActiveCN107935858AGood choiceHigh yieldOrganic compound preparationAmino compound preparationOrganic synthesis2-Nitroaniline
The invention belongs to the field of organic synthesis, and particularly relates to a 5-fluoro-2-nitrophenol preparation method. In the prior art, the existing synthesis method has disadvantages of low conversion rate and long reaction time so as not to easily achieve industrial operation. A purpose of the present invention is to solve the technical problem in the prior art. The technical schemeis to provide a 5-fluoro-2-nitrophenol preparation method, which comprises: a) carrying out a reaction on 2,4-difluoronitrobenzene and NH 3 to obtain 5-fluoro-2-nitroaniline; and b) carrying out a reaction on the 5-fluoro-2-nitroaniline under the actions of sulfuric acid and sodium nitrite to obtain the 5-fluoro-2-nitrophenol. According to the present invention, the 5-fluoro-2-nitrophenol preparation method has advantages of good selectivity, almost no side reaction, high yield, short reaction time, simple operation and easy industrialization.
Owner:LIER CHEM CO LTD +1
Preparation method of 3,3',4,4'-tetraminodiphenyl sulfide
The invention discloses a preparation method of 3,3',4,4'-tetraminodiphenyl sulfide. Orderly through a nucleophilic substitution reaction, a reduction reaction and a neutralization reaction, a desired product is produced from 5-halo-2-nitroaniline, 4-halo-2-nitroaniline or 4-halo-o-dinitrobenzene as a raw material. The preparation method comprises that the raw material and sodium sulfide undergo a nucleophilic substitution reaction to produce a dinitrodiamine intermediate product, then the dinitrodiamine intermediate product and a stoichiometric amount of stannous chloride undergo a reduction reaction to produce hydrochloride of tetramine, then the hydrochloride of tetramine undergoes a neutralization reaction under the action of a sodium hydroxide aqueous solution to produce a crude desired product, and the crude desired product is subjected to column chromatography-based separation purification to form 3,3',4,4'-tetraminodiphenyl sulfide, or comprises that 4-halo-o-diphenylamine as a raw material directly undergoes a nucleophilic substitution reaction to directly produce 3,3',4,4'-tetraminodiphenyl sulfide. The preparation method of 3,3',4,4'-tetraminodiphenyl sulfide has the characteristics of cheap and easily available raw material, short reaction time, simple and easy processes and high yield.
Owner:BEIJING UNIV OF CHEM TECH
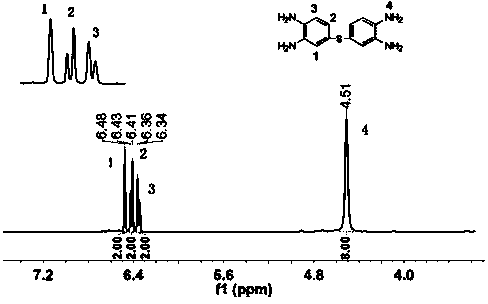
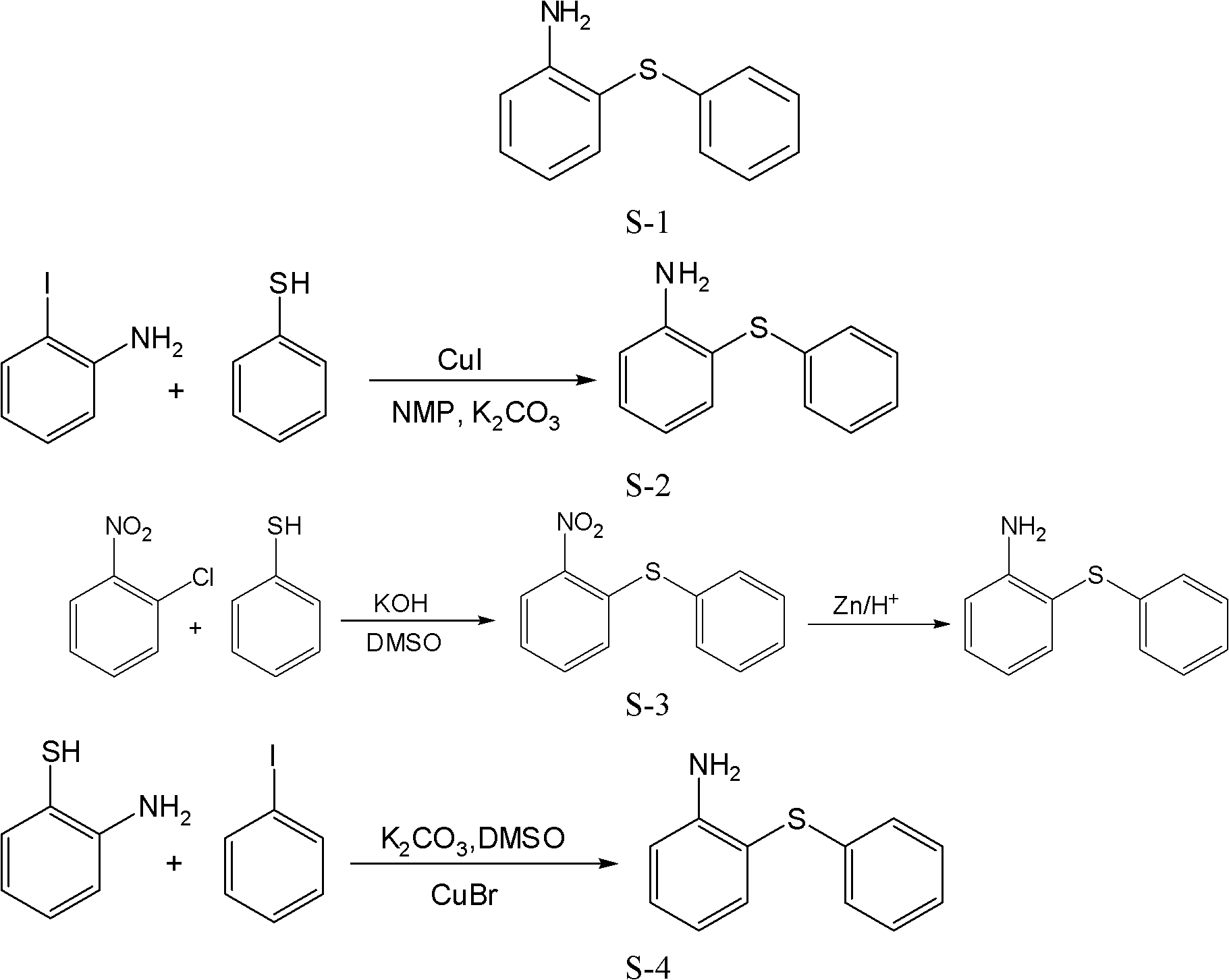
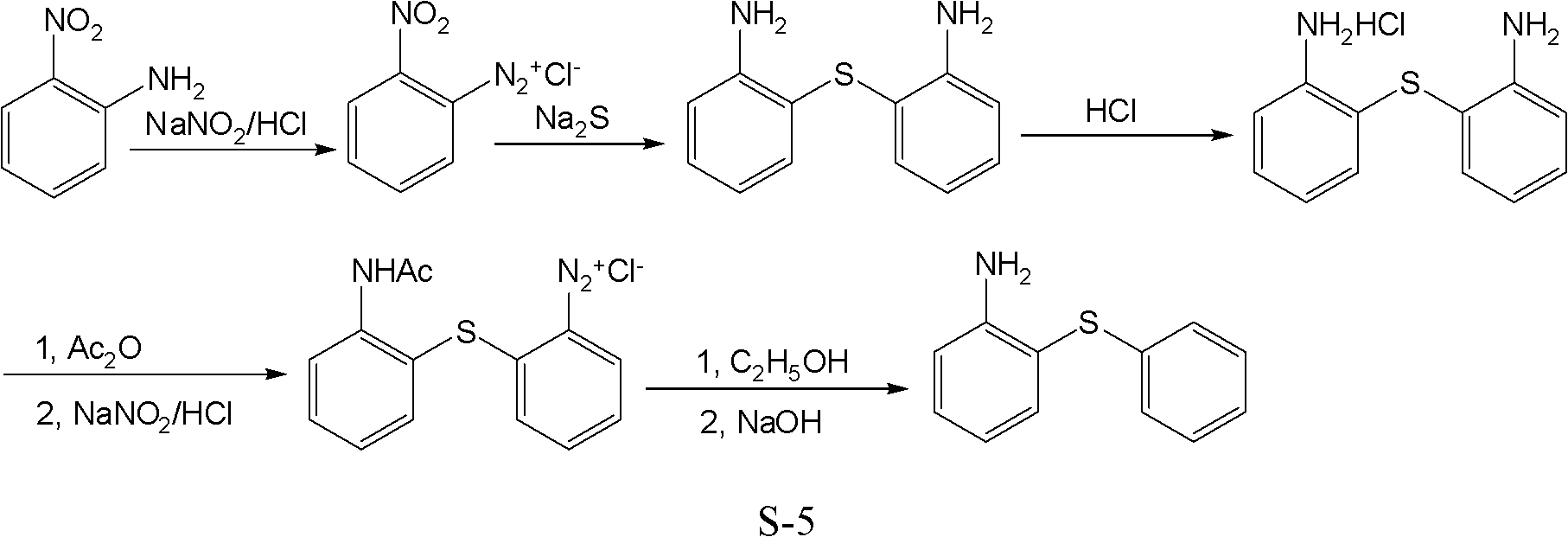
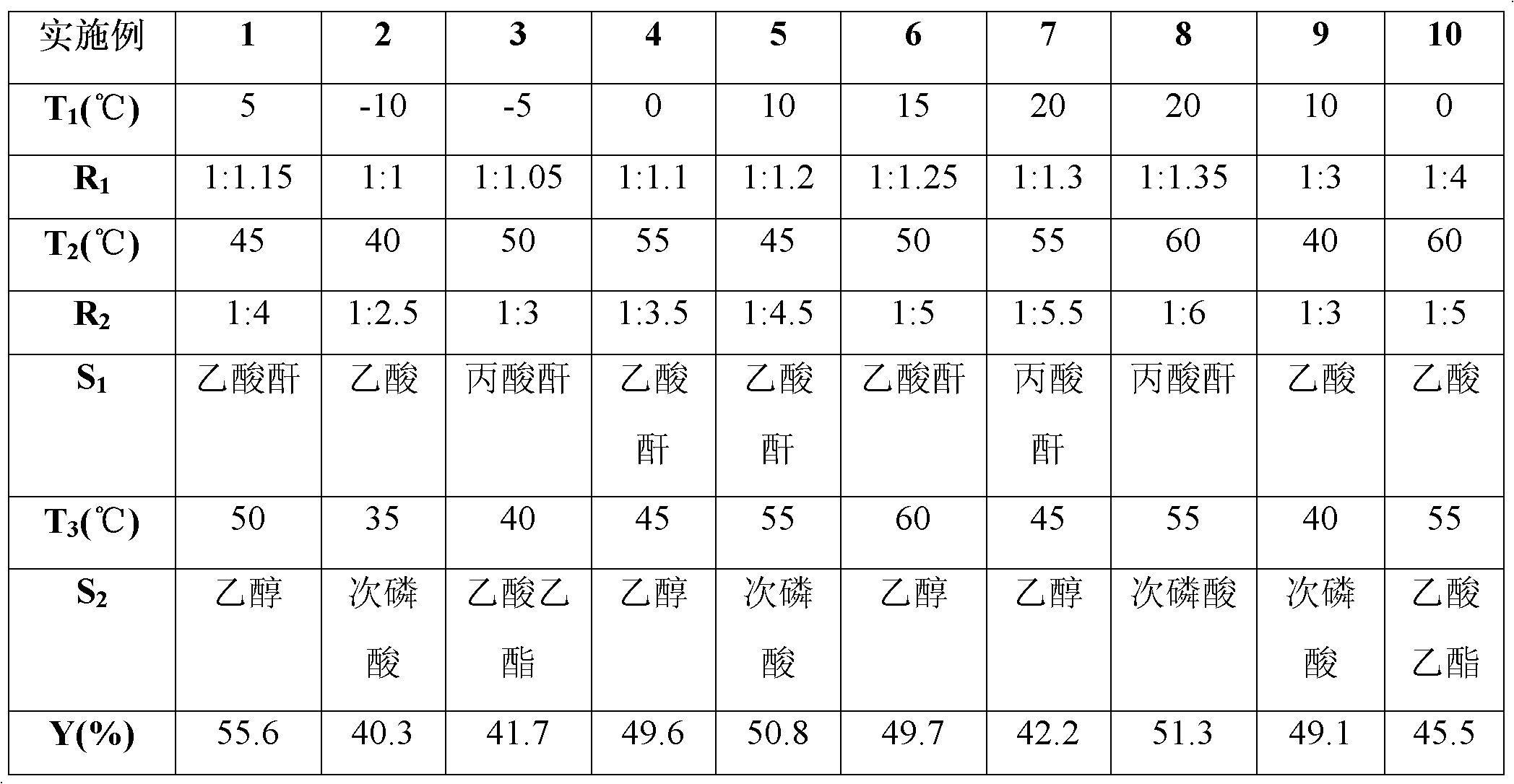
Preparation method of 2-aminophenyl phenyl sulfide
ActiveCN102603584ALow costRaw materials are easy to getSulfide preparationSodium nitriteReducing agent
The invention discloses a preparation method of 2-aminophenyl phenyl sulfide. In the method, a diazo reaction, a thioetherfication-reduction 'one pot process' reaction, a salting reaction, an acylation reaction, a single diazo reaction, a denitriding reaction and a neutralizing reaction are performed in sequence on ortho-nitroaniline serving as a raw material, so that 2-aminophenyl phenyl sulfide is obtained. The method comprises the following steps of: firstly, undergoing a diazo reaction with sodium nitrite and hydrochloric acid, undergoing a thioetherfication-reduction 'one pot process' reaction, i.e., thioetherfication reaction with sodium sulfide, raising the temperature, and undergoing a reduction reaction; secondly, reacting with an equal molar amount of hydrochloric acid for salting, reacting with an acylation reagent to form acylamide, and undergoing a diazo reaction with sodium nitrite and hydrochloric acid; and lastly, undergoing a denitriding reaction under the action of a reducing agent (a denitriding reaction reagent), and neutralizing with an alkaline solution to obtain 2-aminophenyl phenyl sulfide. The method disclosed by the invention for preparing the 2-aminophenyl phenyl sulfide has the characteristic of low cost.
Owner:江西扬帆新材料有限公司 +1
New process synthesis method for 2-sulfydryl-5-methoxybenzimidazole
InactiveCN109879813ALow corrosion resistance requirementsReduce the potential threat level of life hazardOrganic chemistry2-NitroanilineNitrobenzene
The invention discloses a new process synthesis method for 2-sulfydryl-5-methoxybenzimidazole. The new process synthesis method comprises the following steps: preparing N-benzyl-5-methoxy-2-nitroaniline through substitution reaction of 2-fluorine-4-methoxy-1-nitrobenzene and benzylamine; triggering hydrogenation reduction reaction, thereby acquiring 4-metoxybenzene-1,2-diamido; triggering cyclization reaction, thereby acquiring a target product compound 2-sulfydryl-5-methoxybenzimidazole. According to the new process synthesis method for 2-sulfydryl-5-methoxybenzimidazole provided by the invention, the synthetic route method is optimized and improved, operation danger level and production cost are reduced, operation safety is high, aftertreatment is green and environment-friendly, steps and processes are simple, solvent and technological conditions are safe and low-cost, environment-friendly industrial production can be realized and application prospect is bright.
Owner:成都泰蓉生物科技有限公司
Chemical synthesizing method for 5-chlorine-2-nitroaniline
InactiveCN1182104CAtom utilization is highImprove protectionOrganic compound preparationAmino compound preparationChemical synthesis2-Nitroaniline
The method for synthesizing 5-chloro-2-nitrophenylamine uses 3-chlorophenylamine as initial raw material, and utilizes three-step reaction of formylation, nitration and hydrolysis to synthesize object compound 5-chloro-2-nitrophenylamine, its total yield rate is above 60% and its product purity is above 98%.
Owner:ZHEJIANG UNIV OF TECH
Novel photochromic azobenzene compound and synthesis method thereof
InactiveCN104926684ASimple post-processingImprove conversion rateOrganic chemistryEnvironmental resistanceSynthesis methods
The invention provides a novel photochromic azobenzene compound and a synthesis method thereof and relates to a novel compound N-[4-[2-(4-methoxyphenyl) diazenyl] phenyl]-2-nitroaniline with a photochromic property and a synthesis method of the novel compound. The synthesis method comprises diazonium coupling reaction and coupling reaction between aromatic amine and aromatic halides. According to the invention, coupling reaction conditions of aromatic amine and aromatic halides are very simple, to be specific, only alkali metal fluorides with equal molar mass are added into reaction liquid, and reflux is carried out at 130 DEG for 3 h; a solvent is not required to be subjected to anhydrous anaerobic treatment, that reaction is carried out under nitrogen or argon atmosphere is not required, no side reaction is generated, aftertreatment of the product is simple, the conversion rate is higher, the yield of the target product is 48-74%, in addition, equipment adopted by the invention is relatively simple, the cost is low, and obvious economic benefits and environment-protection benefits are realized. The defects, in the prior art, that coupling reaction between aromatic amine and aromatic halides can be carried out at high temperature, with existence of inorganic base and under anhydrous anaerobic inert atmosphere usually, the reaction time is long, side reaction is multiple and the yield is low are overcome.
Owner:HUBEI UNIV
Weft printing dye
The invention discloses weft printing dye. The weft printing dye is composed of the following components, by mass, 70-90% of bottoming liquid and 10-30% of color paste liquid. The bottoming liquid is composed of the following components: 4-4.5% of naphthol AS, 3-3.5% of NaOH with the mass concentration being 30%, 2-2.8% of fatty alcohol-polyoxyethylene ether, 1-1.5% of carbamide, and the balance water. The color paste liquid is composed of the following components: 1-1.3% of methyl-2-nitroaniline, 1.8-2.2% of hydrochloric acid with the mass concentration being 30%, 0.5-0.9% of sodium nitrite, 0.3-0.6% of sodium acetate, 0.9-1.3% of acetic acid, 10-15% of starch-gum tragacanth paste, 9-12% of sodium alginate paste, 2-2.5% of adhesive, and the balance water. Compared with the prior art, the weft printing dye has the advantages that under the action of the moderate adhesive, the sodium alginate paste and the starch-gum tragacanth paste, the adhesiveness of the dye is improved greatly, and prints have good toughness and abrasive resistance.
Owner:JURONG CITY HOUBAI TOWN YINGRUI PRINTING FACTORY
Solar heat absorbing material
InactiveCN107779033AGood adhesionImprove impact resistanceFireproof paintsRadiation-absorbing paintsEpoxy2-Nitroaniline
A solar heat absorbing material comprises resin, a curing agent, an absorbing agent, a solvent, an assistant and an absorbing fortifier. A solar heat absorbing coating is prepared through the following components in parts by weight: 1-3 parts of the mixture of 2-bromo-2-(2-fluorophenyl)-1-cyclopropyl and butanone-2-(2-nitroaniline)-3-cyano-5-thiotolene, 40-60 parts of epoxy modified acrylate resin, 8-12 parts of drying oil modified alkyd resin, 6-8 parts of manganese dioxide, 2-4 parts of ferric oxide, 1-3 parts of titanium dioxide, 10-30 parts of paraxylene, and 5-8 parts of an assistant. According to the solar heat absorbing material, the solar absorbing rate can reach 95% or above, and the transmission rate is 0.19-0.25.
Owner:GUANGXI JIKUAN SOLAR ENERGY EQUIP
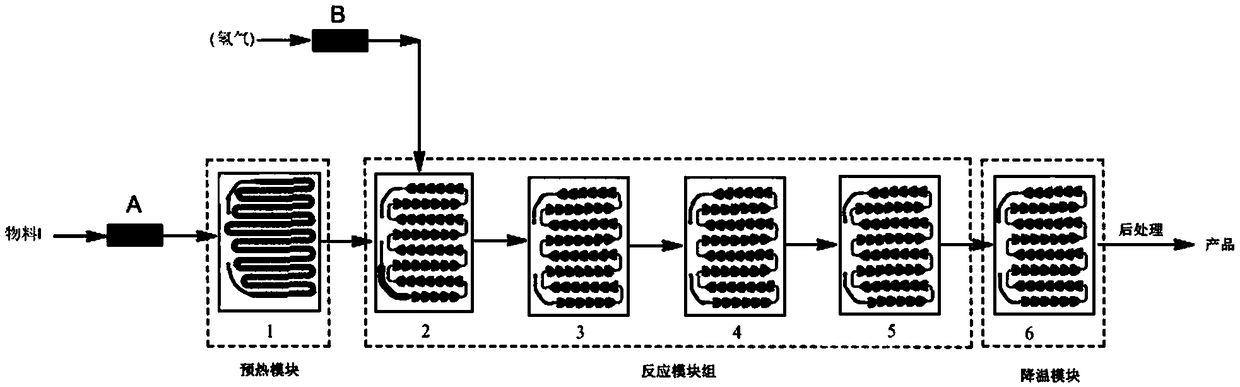
Method for synthesizing dovitnib intermediate in microchannel reactor
InactiveCN108689964AQuick responseReduce energy consumptionOrganic chemistryActivated carbonCombustion
The invention discloses a method for synthesizing a dovitnib intermediate in a microchannel reactor and belongs to the technical field of antitumor drug synthesis in organic synthesis. The method comprises adding 5-(4-methylpiperazine)-2-nitroaniline into an organic solvent, adding an activated carbon-loaded precious metal catalyst into the solution to obtain a mixture as a material I, conveying the material I into a preheating module of the microchannel reactor, carrying out preheating, feeding the preheated material into a reaction module set, feeding hydrogen into the reaction module set ofthe microchannel reactor so that the hydrogen and the preheated material I undergo a reaction in the reaction module set, collecting the reaction liquid flowing out of a cooling module and carrying out post-treatment to obtain the dovitnib intermediate 4-(4-methylpiperazinyl)-1, 2-phenylenediamine. The method can effectively shorten the reaction time, greatly reduce the combustion and explosion safety hazard caused by hydrogen leakage, and is suitable for the process of synthesizing the dovitnib intermediate.
Owner:HEILONGJIANG XINCHUANG BIOLOGICAL TECH DEV CO LTD
Preparation method of bromo-substituted benzimidazole derivative
The invention discloses a preparation method of a bromo-substituted benzimidazole derivative 5-bromo-1,6-dimethyl-1H-benzo[d]imidazolyl-2-formaldehyde, which comprises the following steps: by using 5-bromo-4-methyl-2-nitroaniline as the initial raw material, carrying out reduction, cyclization, methylation and hydrolysis reaction to obtain the target product. The compound is an important drug intermediate.
Owner:湖南华腾制药有限公司
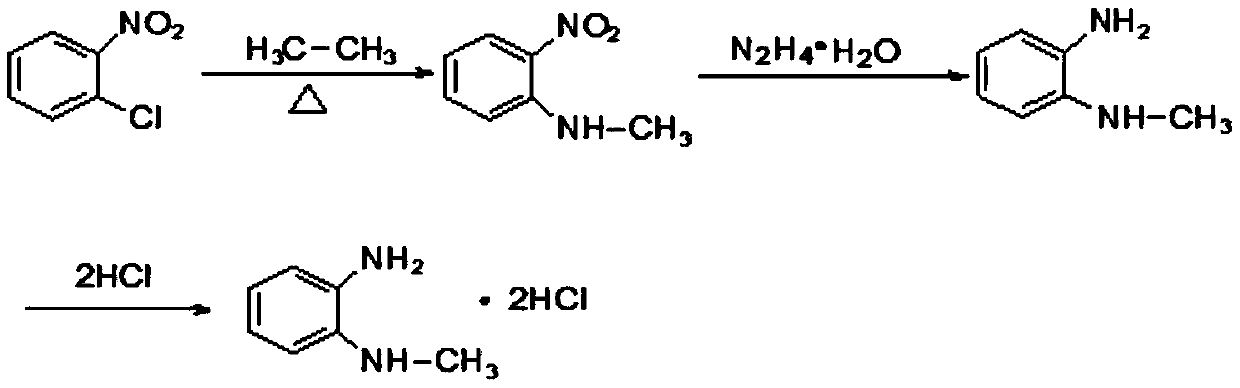
Synthesis method of N-methyl-1,2-benzenediamine dihydrochloride
InactiveCN110272347AHigh purityLess impuritiesOrganic compound preparationAmino compound preparationFiltrationSynthesis methods
The invention discloses a synthesis method of N-methyl-1,2-benzenediamine dihydrochloride. The synthesis method is characterized by comprising the following steps that 1, o-chloronitrobenzene and a monomethylamine aqueous solution are subjected to a sealing reaction, after the reaction is completed, cooling, standing and layering are conducted, and a lower-layer oil phase is collected to obtain N-methyl-2-nitroaniline; 2, a catalyst is added to a mixed solution of ethyl alcohol and the N-methyl-2-nitroaniline obtained in step 1, after mixing and heating, hydrazine hydrate is slowly dropwise added to the mixed solution, and after drop addition is completed, a heat preservation reaction is conducted; after the reaction is completed, suction filtration is conducted, and a suction filtration mother solution is reserved to obtain N-methyl-o-phenylenediamine; 3, liquid caustic soda, water and EDTA are added to the N-methyl-o-phenylenediamine obtained in step 2 for mixing, after cooling is conducted, dichloromethane is added, stirring, extraction, standing and layering are conducted, a lower-layer oil phase is collected, and through separation and purification, the target product N-methyl-1,2-benzenediamine dihydrochloride is obtained. The N-methyl-1,2-benzenediamine dihydrochloride product prepared through the method has high purity and few impurities and can be widely applied to the field of intermediate synthesis of a medicine tishamitan for reducing the blood pressure.
Owner:武汉本杰明医药股份有限公司
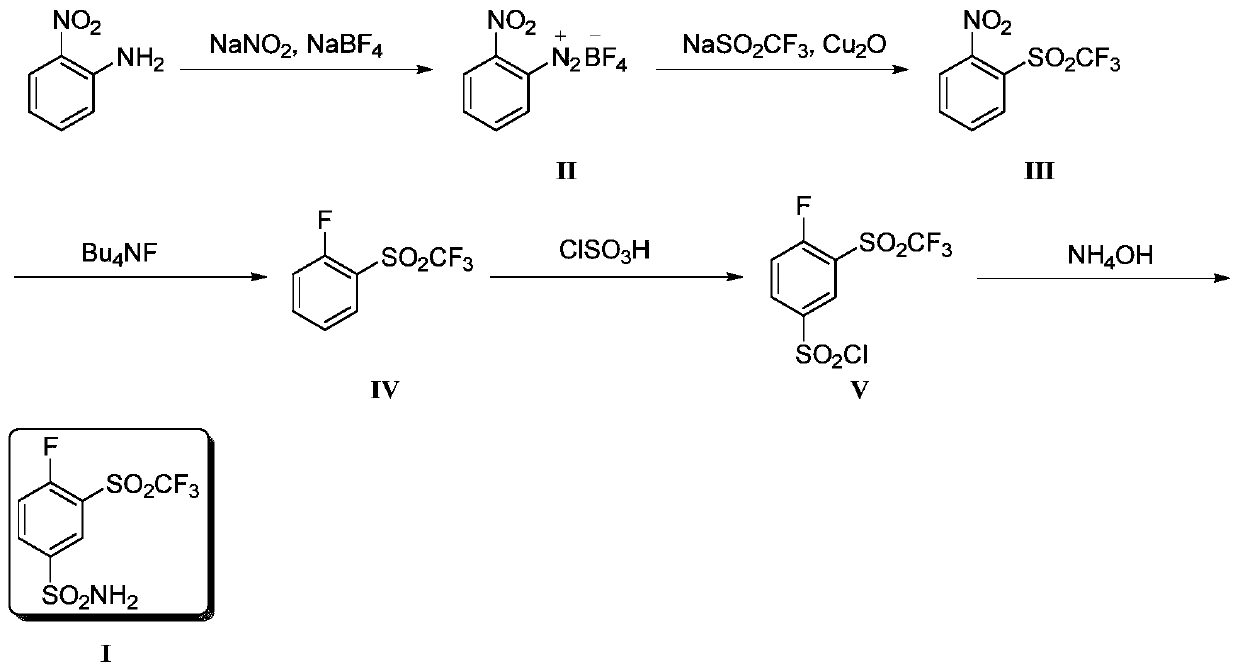
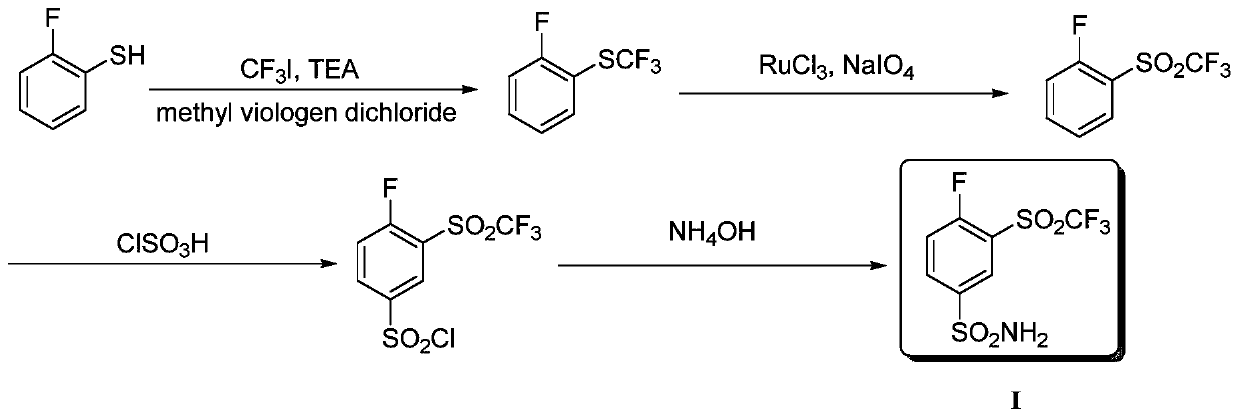
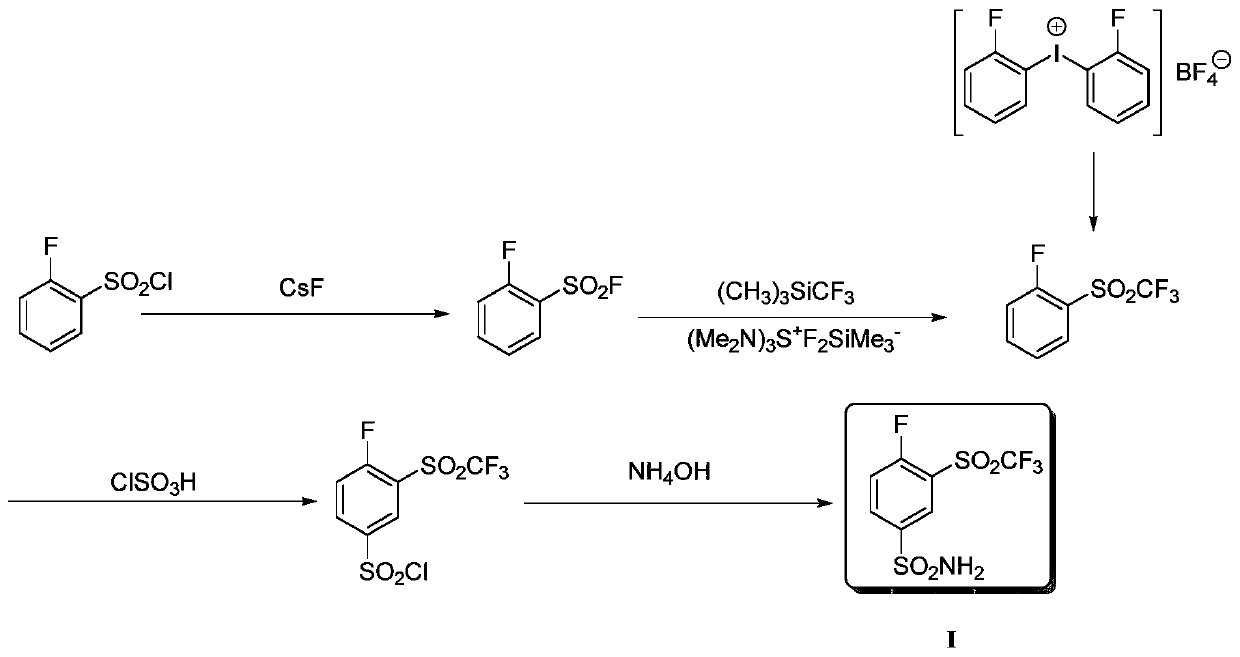
Method for producing 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide
InactiveCN109851535AHigh yieldHigh reaction yieldOrganic chemistryOrganic compound preparationSynthesis methods2-Nitroaniline
The invention provides a method for producing 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide. 2-nitroaniline is used as an initial raw material, is subjected to diazotization reaction, trifluoromethyl sulfonation reaction, fluoronation, chlorosulfonation reaction, and is reacted with ammonium hydroxide to finally produce the 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide. Comparedwith reported synthesis methods, the method for producing the 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide has the advantages that the reaction yield of the synthesis route is high, used reaction agents are economic and easy to obtain, reaction conditions are mild and controllable, and in a reaction process, column chromatography and purification are not needed, so that the method for producing the 4-fluoro-3-(trifluoromethylsulfonyl)benzenesulfonamide has wide commercial application prospects.
Owner:TIANJIN MODERN VOCATIONAL TECH COLLEGE
Synthesis method of triclabendazole
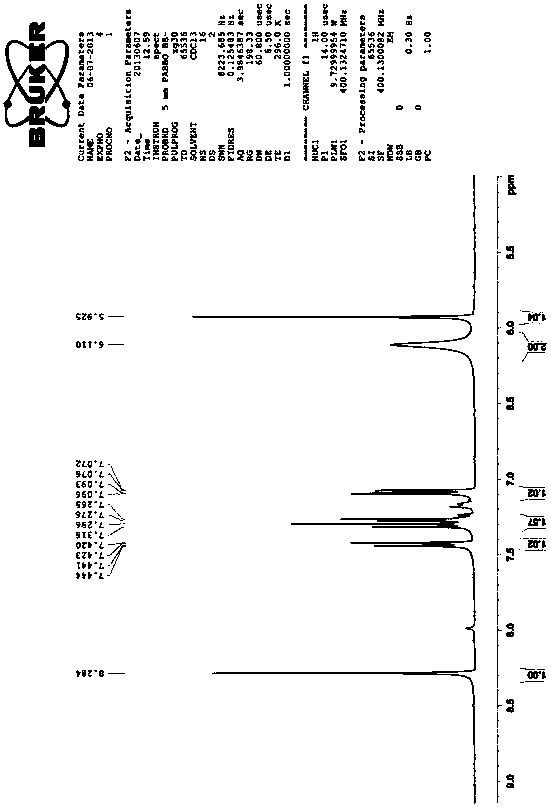
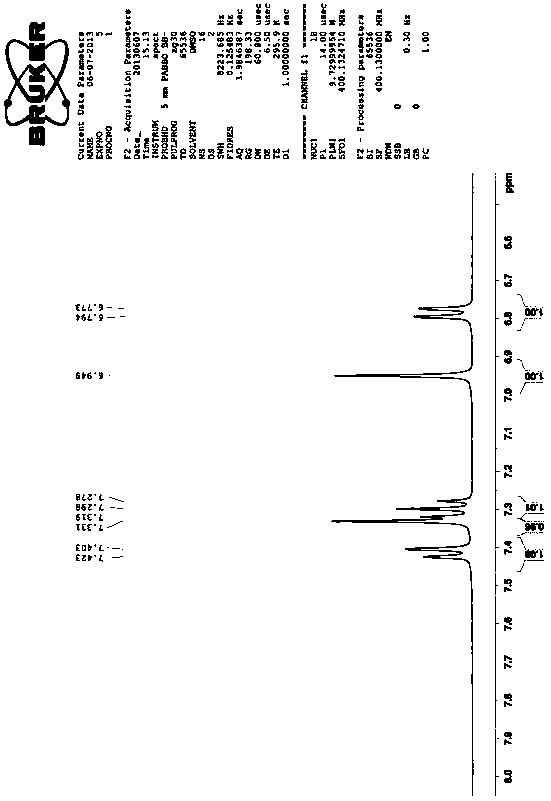
The invention discloses a synthesis method of triclabendazole, comprising the following steps: reducing 4-chloro-5-(2,3-dichlorophenoxy)-2-nitroaniline as a raw material in an organic solvent by trichlorosilane, thus obtaining an intermediate compound; directly making ring closing reaction with carbon disulfide in the presence of potassium hydroxide, thus preparing an intermediate; enabling the intermediate and dimethyl carbonate to make methylation reaction under DBU catalysis, thus finally preparing triclabendazole. Through the process, a nitro reduction method is improved, the requirementsfor equipment are reduced, and post-treatment steps are simplified; by combining the nitro reduction and the cyclization into a one-step reaction, the operation procedure is simplified and the yield is increased; by changing a feeding sequence in the process of cyclization, the release rate of hydrogen sulfide is controlled, and the influence on environment is reduced. Replacing dimethyl sulfate or methyl iodide with dimethyl carbonate for methylation is more environmentally friendly; the obtained triclabendazole meets the European Pharmacopeas Standards in terms of all indicators, the total yield can reach 55-60%, indicating an obviously improved process and a promising prospect in industrial application.
Owner:暨明医药科技(苏州)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com