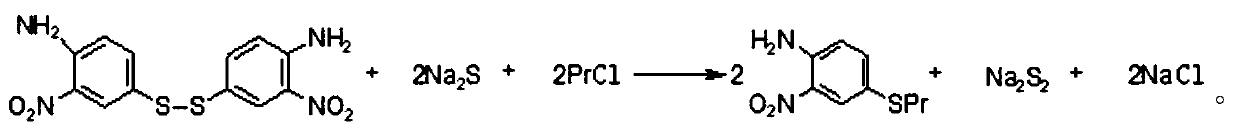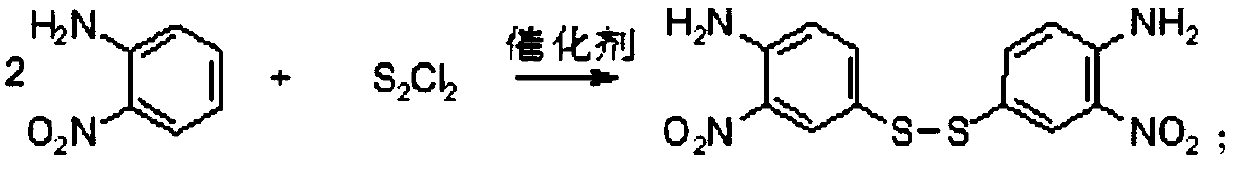Preparation method of 4-propythio-2-nitroaniline
A technology of nitroaniline and o-nitroaniline, which is applied in the field of preparation of 4-propylthio-2-nitroaniline, can solve the problems of affecting the economic benefits of industrial production, poor industrialization feasibility, complicated operation steps, etc. Low, easy to purify, simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The method for preparing 4-propylthio-2-nitroaniline, comprises the following steps:
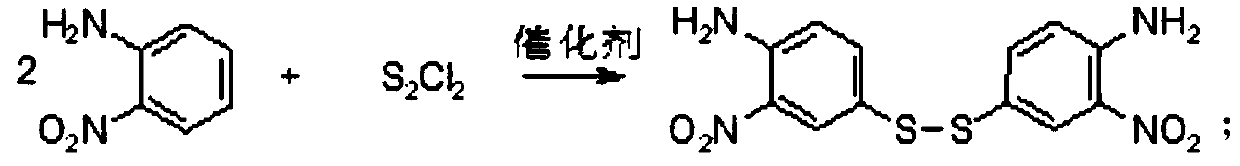
[0028] (1) Condensation reaction: Add methanol 120g, o-nitroaniline 30g, disulfur dichloride 30.80g, acidified active clay / pseudoboehmite composite catalyst (mass ratio 1:1) 1.5 g is the catalyst. It was condensed at 25° C. for 3 hours to form bidisulfide. The catalyst was filtered for further use. 36.09 g of bidisulfide was obtained by separation, and the condensation reaction yield was 98.24%.
[0029] (2) Alkylation reaction: Add 36.09g of bidisulfide and 72.17g of methanol prepared in step (1) to a 250ml four-necked bottle, add 56.37g of sodium sulfide nonahydrate, keep warm for 30min, and control the temperature at 55 18.30 g of chloropropane was added dropwise at ℃ to generate 4-propylthio-2-nitroaniline, and 44.38 g of 4-propylthio-2-nitroaniline was obtained by isolation, and the yield of the alkylation reaction was 98.05%.
[0030] The total yield of 4-propylthio-2-nitroanil...
Embodiment 2
[0032] The method for preparing 4-propylthio-2-nitroaniline, comprises the following steps:
[0033] (1) Condensation reaction: Add methanol 120g, o-nitroaniline 30g, disulfur dichloride 29.33g, acidified active clay / pseudoboehmite composite catalyst (mass ratio 1:1) 1.5 g is the catalyst. It was condensed at 25° C. for 3 hours to form bidisulfide. The catalyst was filtered for further use. 33.43 g of bidisulfide was obtained by separation, and the condensation reaction yield was 91.01%.
[0034] (2) Alkylation reaction: Add 33.43g of bidisulfide and 66.86g of methanol prepared in step (1) into a 250ml four-necked bottle, add 52.23g of sodium sulfide nonahydrate, keep warm for 30min, and control the temperature at 55 16.96 g of chloropropane was added dropwise at ℃ to react to generate 4-propylthio-2-nitroaniline. 40.10 g of 4-propylthio-2-nitroaniline was isolated, and the yield of the alkylation reaction was 95.64%.
[0035] Preparation of 4-propylthio-2-nitroaniline react...
Embodiment 3
[0037] The method for preparing 4-propylthio-2-nitroaniline, comprises the following steps:
[0038] (1) Condensation reaction: Add methanol 120g, o-nitroaniline 30g, disulfur dichloride 30.80g, acidified activated clay / pseudoboehmite composite catalyst (mass ratio 1:1) 0.75 g is the catalyst, which is condensed at 25° C. for 3 hours to form bidisulfide. The catalyst is filtered for further use, and 28.15 g of bidisulfide is obtained by separation, with a condensation reaction yield of 76.64%.
[0039] (2) Alkylation reaction: Add 28.15 g of disulfide and 56.30 g of methanol prepared in step (1) to a 250 ml four-necked bottle, add 43.98 g of sodium sulfide nonahydrate, keep warm for 30 minutes, and control the temperature at 55 14.28 g of chloropropane was added dropwise at ℃ to react to generate 4-propylthio-2-nitroaniline. 34.44 g of 4-propylthio-2-nitroaniline was isolated, and the yield of the alkylation reaction was 97.55%.
[0040] The total yield of the reactions for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com