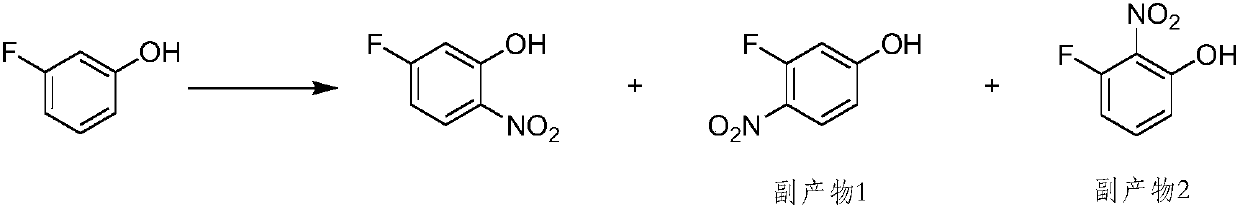
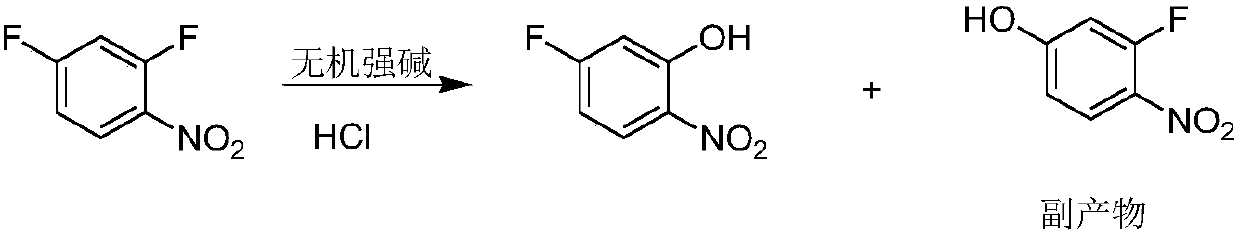
5-fluoro-2-nitrophenol preparation method
A technology of nitrophenol and difluoronitrobenzene is applied in the field of preparation of 5-fluoro-2-nitrophenol, can solve the problems of unfavorable industrial operation, low conversion rate of synthesis method, long reaction time and the like, and achieves the reaction time Short, easy to operate, no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
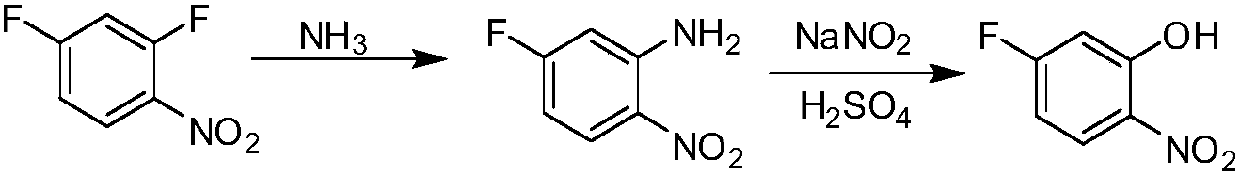
[0025] The preparation method of 5-fluoro-2-nitrophenol comprises the following steps:
[0026] a) 2,4-difluoronitrobenzene and NH 3 Reaction to obtain 5-fluoro-2-nitroaniline;
[0027] b) Dissolving 5-fluoro-2-nitroaniline in sulfuric acid aqueous solution first, then adding sodium nitrite aqueous solution dropwise to the above aqueous solution at 0°C-10°C, and then reacting at 0°C-10°C for 0.5-1h, Then within 1~2h, the temperature was raised to 90~95°C for 1h.
[0028] As a preferred solution of the present invention, in the preparation method of the above-mentioned 5-fluoro-2-nitrophenol, the NH described in step a) 3 For concentrated ammonia.
[0029] In the preparation method of the above-mentioned 5-fluoro-2-nitrophenol, the NH described in step a) 3 The molar ratio to 2,4-difluoronitrobenzene is 2~4:1. Preferably, the NH 3 The molar ratio to 2,4-difluoronitrobenzene is 2.1~2.5︰1.
[0030] In the above-mentioned preparation method of 5-fluoro-2-nitrophenol, the re...
Embodiment 1
[0038] Synthesis of embodiment 1 5-fluoro-2-nitroaniline
[0039] Add 127.5 g of concentrated ammonia water into a 500 mL reaction flask, and add 159 g (1 mol) of 2,4-difluoronitrobenzene at room temperature. Start stirring, and slowly raise the temperature to 40°C for 3 hours. After the raw materials disappeared in the central control analysis, it was cooled to 5-10°C under stirring to crystallize, and 152.9 g of 5-fluoro-2-nitroaniline was obtained by filtration, with a yield of 98%.
Embodiment 2
[0040] Example 2 Synthesis of 5-fluoro-2-nitroaniline
[0041] Add 180g of water into a 500mL reaction flask, and add 159g (1mol) of 2,4-difluoronitrobenzene at room temperature. Turn on the stirring, feed 42.5 g of ammonia gas at 35-40°C, and then keep it warm for 3 hours. After the raw materials disappeared in the central control analysis, it was cooled to 5-10°C under stirring to crystallize, and 153.2 g of 5-fluoro-2-nitroaniline was obtained by filtration, with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com